Method for quickly and sensitively detecting etomidate in blood
A technology for etomidate and blood is applied in the field of detecting etomidate in blood, which can solve the problems of inaccurate monitoring of etomidate blood concentration, time-consuming and laborious monitoring by HPLC, and inability to real-time online measurement, so as to ensure data repeatability. , The device is stable and avoids the effect of daytime difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The experimental conditions used in the experiment are: the ionization source is the ion mobility spectrum of lamp ionization, the positive ion mode high voltage source, and the stepping motor thermal analysis sampler. During the experiment, the temperature of the migration tube was kept at 100°C, the sampler was 200°C, and the flow of carrier gas (air) and bleaching gas (air) were 300 mL / min and 400 mL / min, respectively. The internal standard is DNP, 10ng / μl, the sample injection volume is 10μl, and 10μl of the internal standard is mixed and dried at room temperature (20-30℃).
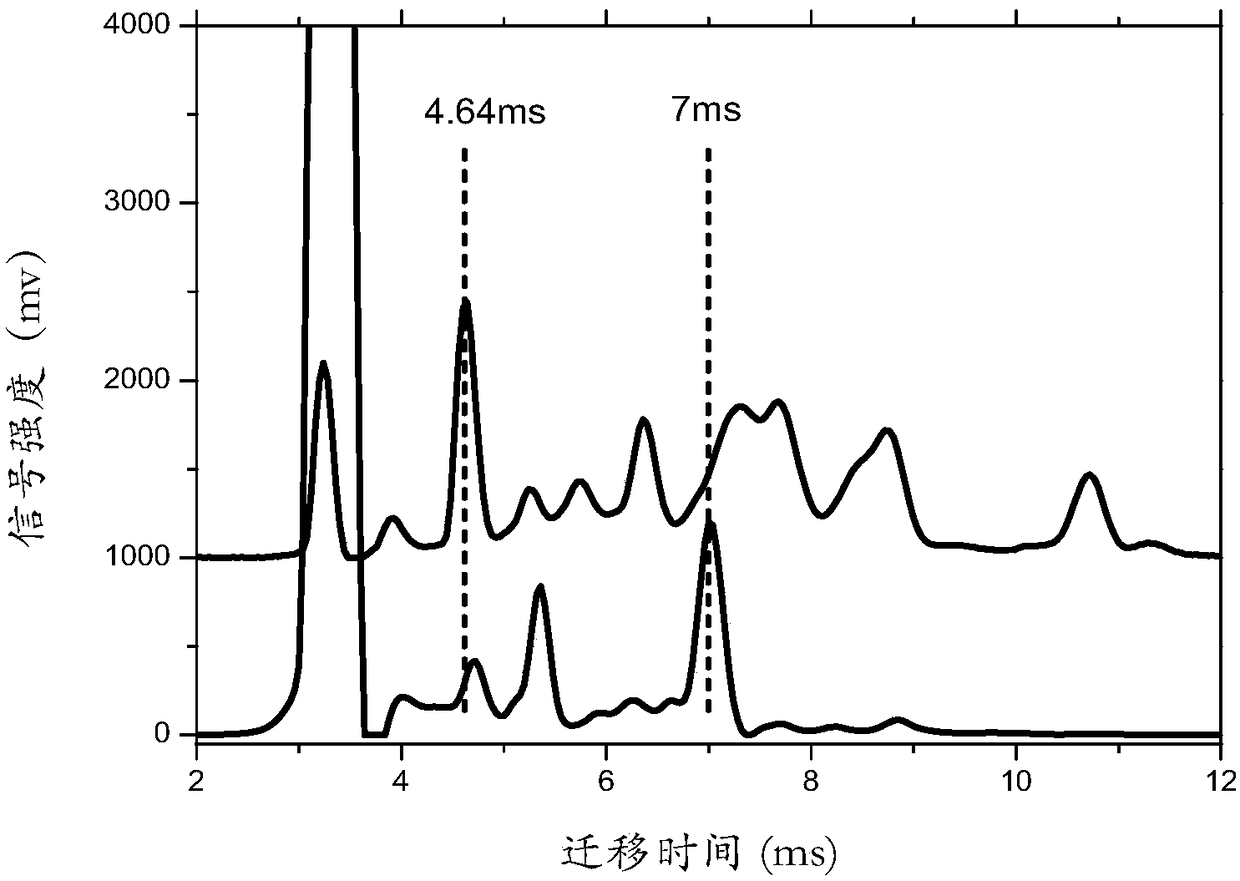
[0031] figure 1 The ion mobility spectrum of etomidate detected in positive ion mode is given.
[0032] Such as figure 1 As shown, it can be known by ion mobility calibration. When the migration time of acetone is 3.24ms, the migration time of etomidate monomer peak is 4.64ms, and the migration time of internal standard DNP is 7ms. Follow-up tests use this as a standard for qualitative analysis.
Embodiment 2
[0034] The experimental conditions used in the experiment are: the ionization source is the ion mobility spectrum of lamp ionization, the positive ion mode high voltage source, and the stepping motor thermal analysis sampler. During the experiment, the temperature of the migration tube was kept at 100°C, the sampler was 200°C, and the flow of carrier gas (air) and bleaching gas (air) were 300 mL / min and 400 mL / min, respectively. The internal standard is DNP, 10ng / μl, the sample injection volume is 10μl, and 10μl of the internal standard is mixed and dried at room temperature (20-30℃). Etomidate was formulated into samples containing 0.5, 1, 2.5, 5, 12.5 ng / μl etomidate.
[0035] figure 2 The standard curve of etomidate quantitative analysis in positive ion mode is given.
[0036] According to the migration time of etomidate and the internal standard reagent, the ion mobility spectrum was continuously collected for 30 seconds, and the ratio of the peak intensity of the continuous ...
Embodiment 3
[0038] The experimental conditions used in the experiment are: the ionization source is the ion mobility spectrum of lamp ionization, the positive ion mode high voltage source, and the stepping motor thermal analysis sampler. During the experiment, the temperature of the migration tube was kept at 100°C, the sampler was 200°C, and the flow of carrier gas (air) and bleaching gas (air) were 300 mL / min and 400 mL / min, respectively. The internal standard is DNP, 10ng / μl, the sample injection volume is 10μl, and 10μl of the internal standard is mixed and dried at room temperature (20-30℃).
[0039] Determine the signal peak and internal standard peak of the etomidate drug in the blood sample to be tested according to the signal peak migration time obtained by the etomidate drug and the internal standard detection, and bring the maximum signal intensity ratio into the standard curve equation to further determine the blood The content of etomidate in the drug.
[0040] For example, an un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com