Methods and systems for determining fetal spinal muscular atrophy gene haplotypes
A technique for muscular dystrophy and haplotype, applied in the fields of genomics, biochemical equipment and methods, biostatistics, etc., which can solve the problem of the small number of detection sites, the inability to detect maternal mutations, the need for sample size, and the difficulty of sampling, etc. problem, to achieve the effect of simple sample, avoid bleeding, and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
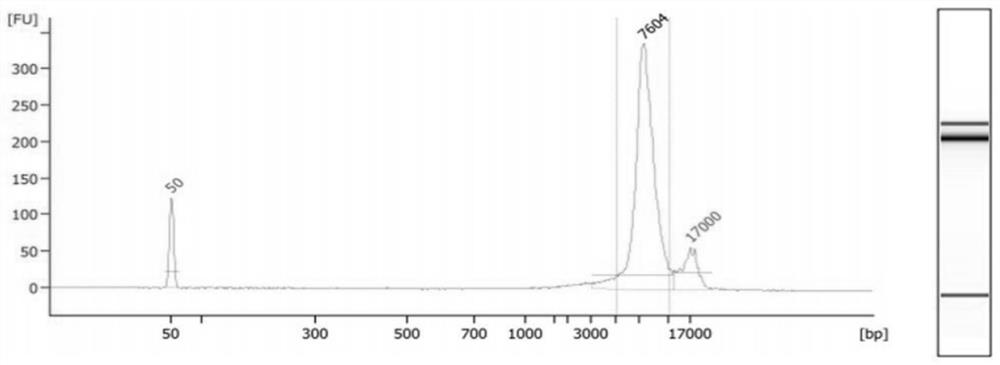
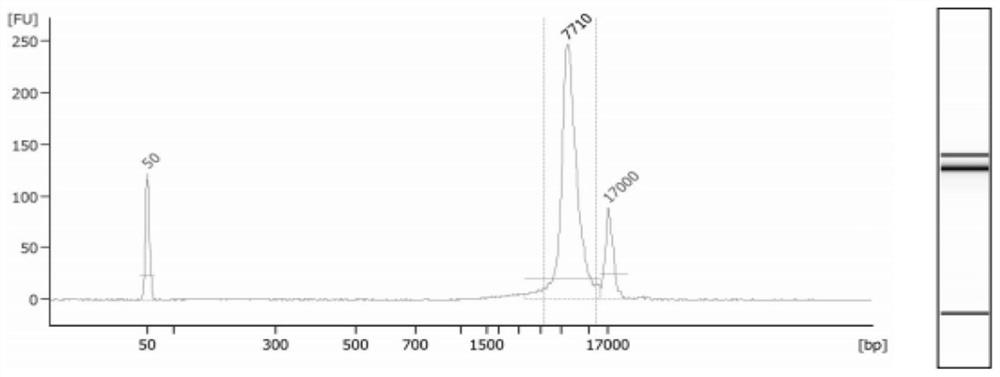
[0172] 1. Preparation of Microarrays Captured by Hybridization of Spinal Muscular Atrophy Gene
[0173] The region where the common spinal muscular atrophy is located, the preferred region on chromosome 5 70,922,133 to 70,955,823 is the spinal muscular atrophy region.
[0174] To remove duplicated fragments, the preferred method is to remove the regions with more than 200 repeated fragments, that is, if a certain fragment can be compared with more than 200 regions on the chromosome, then these fragments are removed.
[0175] To obtain a chip for SMA hybridization capture, the preferred method is a liquid phase capture chip.
[0176] Amplification and enrichment of long fragments can be performed at the same time using the PCR method, which can be used as a supplementary method to assist the chip to capture the target region.
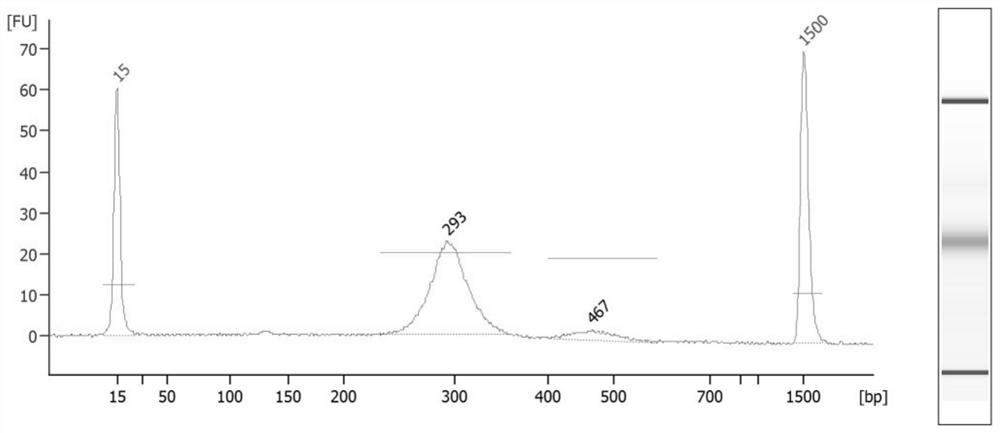
[0177] 2. Construction of the three-generation library
[0178] a. Sample preparation: Obtain fetal paternal and maternal blood samples, and extract w...
Embodiment 2
[0253] One patient was tested for noninvasive SMA. In this case, both the father and mother were carriers of the c.56delT mutation, and the fetus was homozygous for the c.56delT mutation.
[0254] 1. Collection and processing of samples from father and mother
[0255] Streck blood collection tubes were used to collect 5 mL of peripheral blood from the father and mother according to the standard peripheral blood collection operation. After the collection was completed, the peripheral blood of the father and mother was plasma separated in time according to the standard two-step centrifugation method.
[0256] 1.1 Plasma DNA extraction
[0257] The TIANamp Micro DNA Kit was used to extract cell-free DNA from the peripheral blood plasma of the mother. The specific operation steps are as follows:
[0258] 1.1.1 Take 600μL of maternal peripheral blood plasma into a 2mL centrifuge tube, add 20μL of Proteinase K solution, mix well, and centrifuge briefly.
[0259] 1.1.2 Add 600 μL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com