High-efficient expression of recombined swine IL 22 in escherichia coli and its application
A technology of porcine interleukin and Escherichia coli, which is applied in the field of biotechnology and genetic engineering, can solve problems such as unclear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
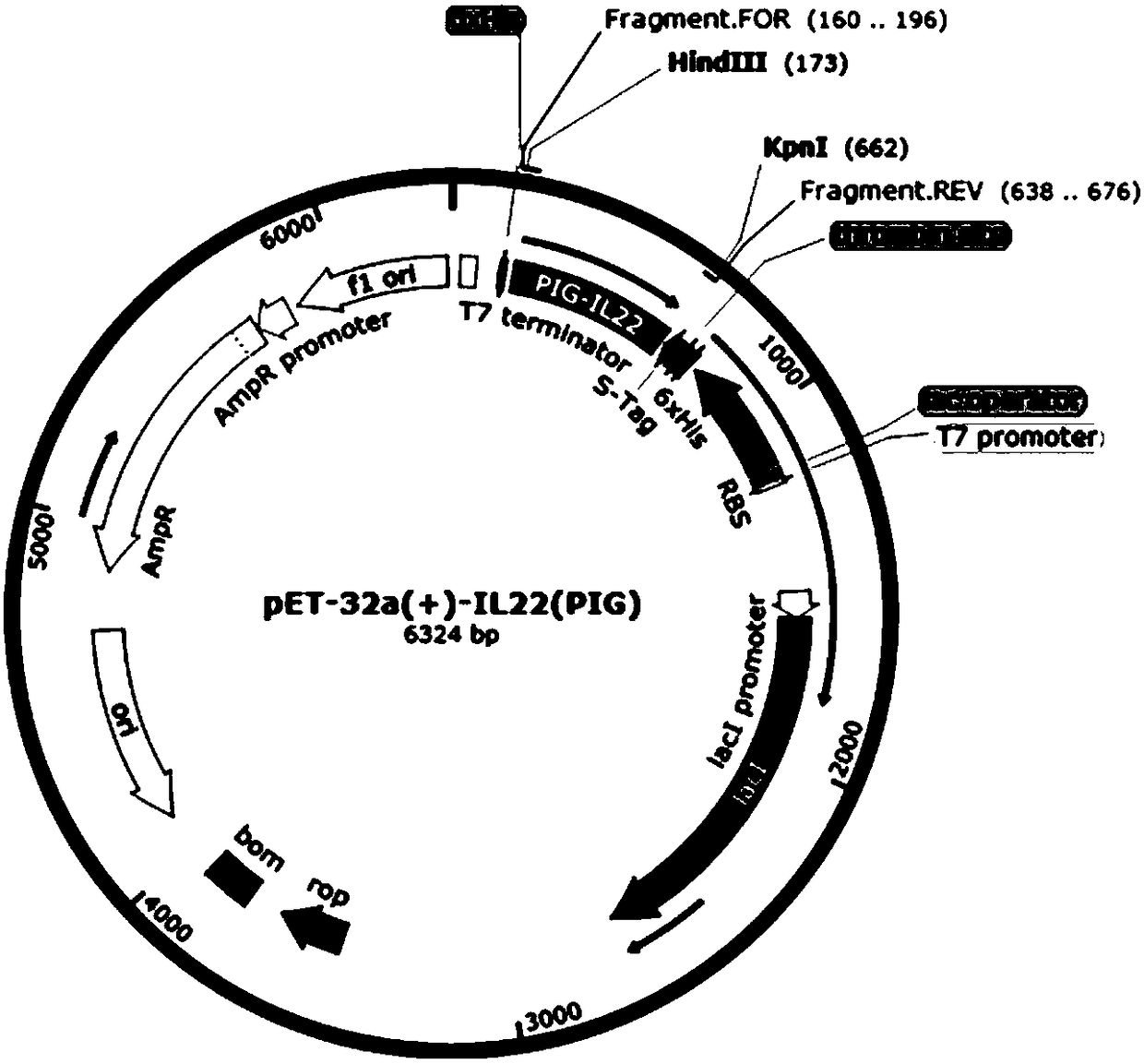
[0048] Embodiment 1 Construction of recombinant plasmid pET-32a(+)-pIL-22
[0049] 1. Obtaining the recombinant porcine interleukin 22 gene fragment
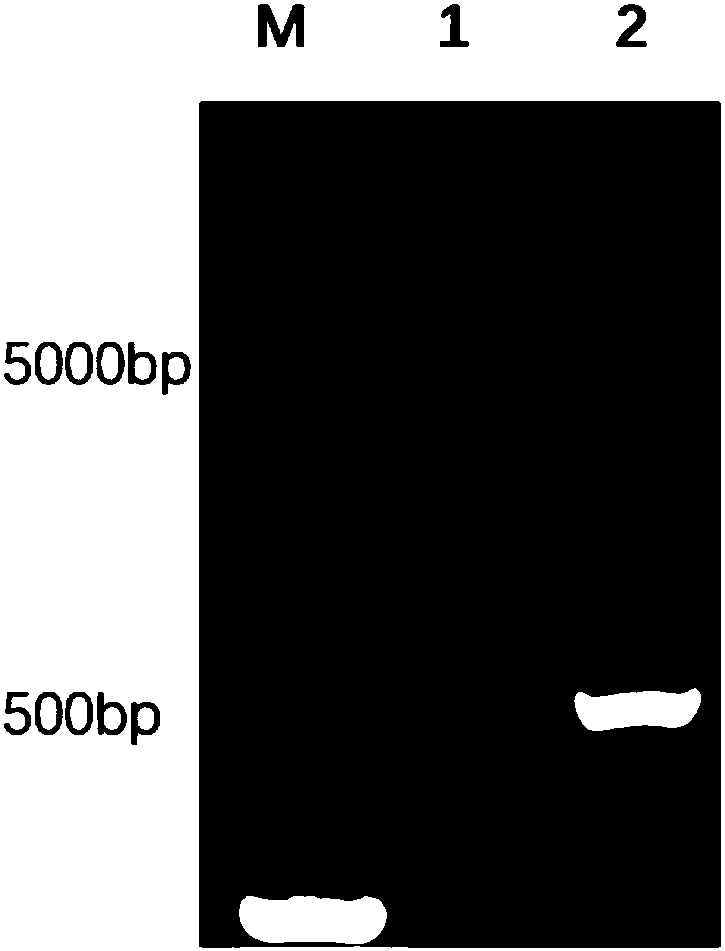
[0050] OptimumGene was used with reference to the published gene sequence (GenBank accession number: KX588234.1) TM Gene design software, according to the preference of the host Escherichia coli E.coli BL21, remove the rare codons (AGA, GGA, CCC, CTA) of Escherichia coli, adjust the GC content of the sequence, optimize the codons, and design the recombinant porcine interleukin The 22 gene sequence is SEQ ID NO: 1, and then the homology arm CAGCCCAGATCTGG of the pET-32a(+) vector linearized by Kpn Ⅰ and Hind Ⅲ enzymes is added to the two ends of the SEQ ID NO: 1 gene sequence GTACC (SEQ ID NO:2) and CGAGTGCGGCCGCA AGCTT (SEQID NO: 3) (the underline is the enzyme cleavage site), the sequence was artificially synthesized (synthesized by Nanjing GenScript Biotechnology Co., Ltd.) to obtain the target fragment of the recombinant ...
Embodiment 2
[0053] Example 2 Verification of expressing porcine interleukin 22 recombinant Escherichia coli E.coli BL21 (DE3)
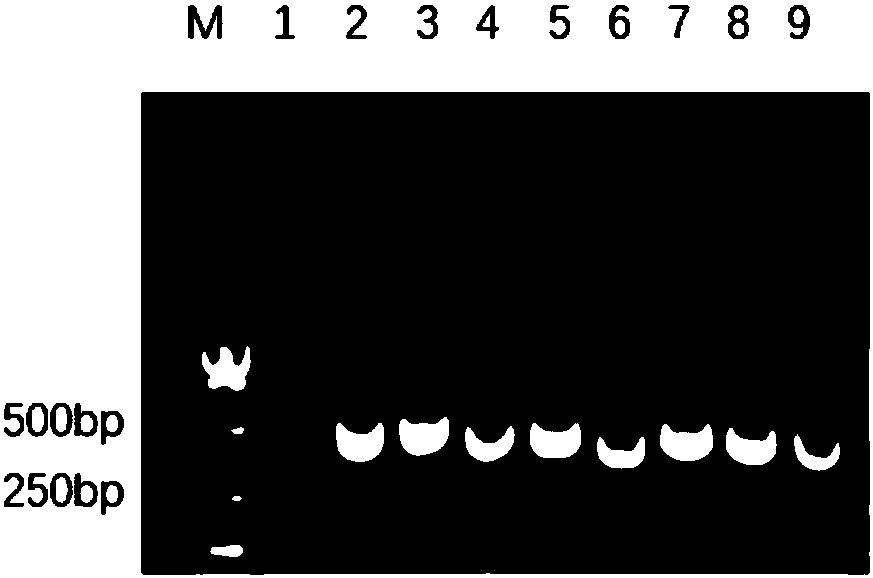
[0054] Take 1 μL (200ng / μL) of the recombinant plasmid pET-32a(+)-pIL-22 and add it to 50 μL of E. coli BL21(DE3) chemical transformation competent cells, flick it a few times, do not suck and blow, and put it on ice Leave it for 30min. Heat shock at 42°C for 90s in a water bath, incubate on ice for 5min, add 900μL of SOC medium, mix gently by inversion, put into a 37°C constant temperature incubator to recover for 10min, and place the recovered bacterial solution in a shaker at 37°C , 150rpm, cultivated for 40min, took out the bacterial solution, centrifuged at 5000rpm for 5min, discarded the supernatant, took 100μL of the bacterial solution and spread it on the ampicillin-resistant solid basal medium LB plate, and positive colonies could be seen in 12-16h. Pick colonies for verification, such as image 3 , 2-9 are colonies verified by PCR, the results show th...
Embodiment 3
[0058] Example 3 Extraction and Purification of Recombinant Porcine Interleukin 22 Expressed by Recombinant Escherichia coli E.coli BL21 (DE3)
[0059] The positive colony of the recombinant E. coli BL21 (DE3) obtained in Example 2 was picked and cultured overnight in LB medium containing ampicillin (50 μg / mL). Inoculate the overnight cultured bacterial solution in a new LB medium at a ratio of 1:100 for cultivation. When the OD value of the bacterial solution reaches 0.5, add IPTG to induce the expression of porcine interleukin-22 protein in recombinant E. coli, and induce at 15°C Two conditions were cultivated for 16h and induced for 4h at 37°C. 8000rpm, 10min, centrifuge to collect the bacteria. PBS was added to resuspend the bacteria collected by centrifugation, and the resuspension was ultrasonically disrupted. After crushing, centrifuge at 14000rpm for 30min. Collect the supernatant and precipitate for SDS-PAGE and Western blot verification. The results show that the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com