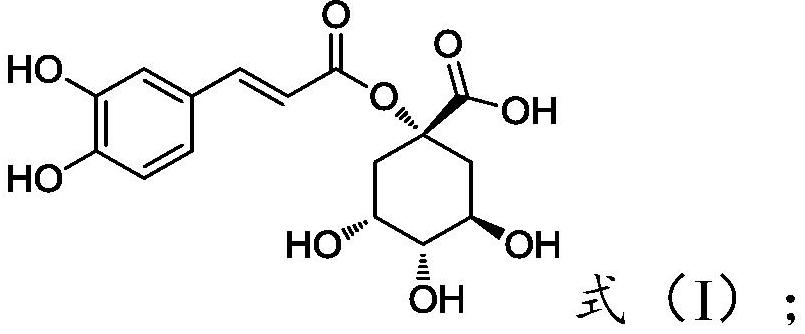
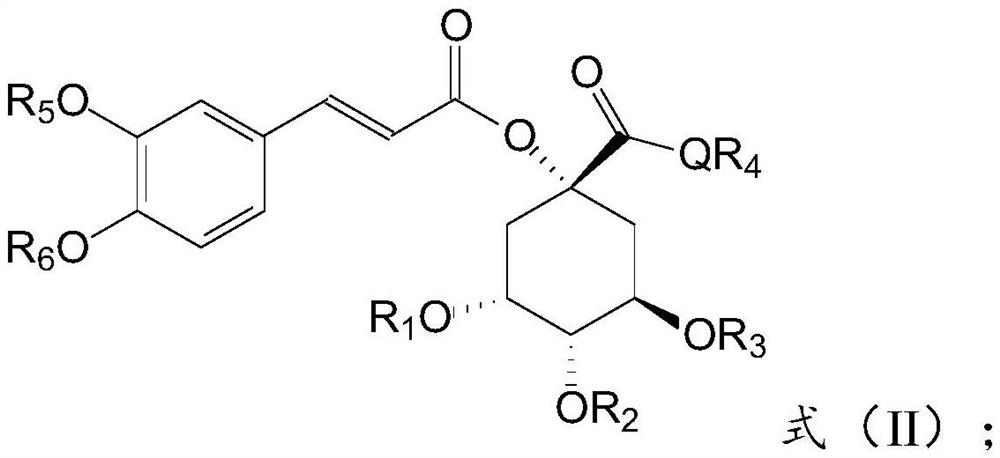
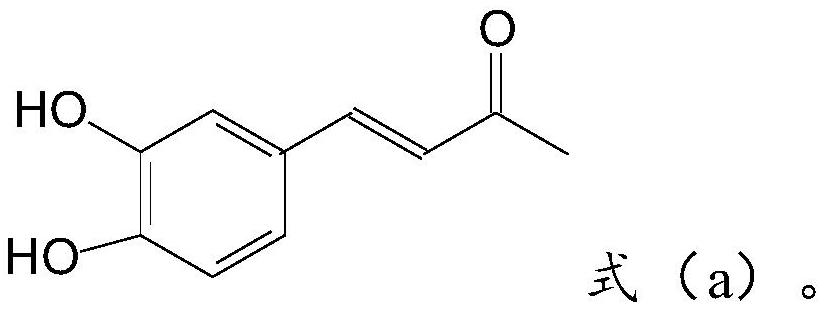
1-o-caffeoylquinic acid, its derivatives, preparation method and use thereof
A technology of caffeoylquinic acid and its derivatives, which is applied in the field of 1-O-caffeoylquinic acid, preparation, and its derivatives, and can solve the problems of not describing the synthesis conditions and yield in detail
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: (1S,3R,4R,5R)-1-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl]oxy]-3,4,5- Preparation of Trihydroxycyclohexanecarboxylic Acid (Compound 1-1)
[0117]
[0118] Step B:
[0119] Add caffeic acid (36.0g, 0.2mol) into the reaction flask, add 1mol / L sodium hydroxide aqueous solution (440ml) to dissolve, cool the system to 0°C, slowly add acetic anhydride (80ml, 0.84mol) dropwise, and keep at 0°C React for 1 h, filter, wash the filter cake until neutral, recrystallize the crude product with ethanol, and dry to obtain 44.87 g of SM white powder, yield: 84.98%, mp: 182-184 °C.
[0120] Step A:
[0121] D-(-)-Quinic acid (30.0g, 0.156mol, according to the literature Valentina Sinisi.Et al., "Interaction of chlorogenic acids and quinides from coffee with human serum malbumin", Food Chemistry, 2015 (168): 332-340 Method preparation), p-toluenesulfonic acid monohydrate (1.50g, 7.8mmol), acetone (1200.0ml) were put into a one-necked bottle, and a Soxhlet extractor with...
Embodiment 2
[0128] Embodiment 2 (preparation of compound 1-4)
[0129]
[0130] Step A:
[0131] Add intermediate 1b (10.0g, 21.72mmol) and DCM (100.0ml) successively to a 100ml three-necked flask, stir to dissolve, then add H 2 O (20.0ml), and trifluoroacetic acid (37.0ml) was slowly added dropwise in an ice-water bath, stirred at room temperature for 5min-15min after the dripping, and the reaction was monitored by sampling TLC. Concentrate the reaction system under reduced pressure with an oil pump at room temperature, add trifluoroacetic acid to toluene, and obtain 7.2 g of yellow foamy solid 1-4a.
[0132] Step B:
[0133] Under the protection of nitrogen, the intermediate 1-4a (5.0g, 11.89mmol) was added to the reaction flask, anhydrous DMF (50.0ml) was added to dissolve, the system was cooled to 0°C, and sodium hydride (1.2g, 29.72mmol) was added in batches , 60% mineral oil), stirred for 30min, slowly added dropwise a DMF solution (10.0ml) containing 2-bromopropane (3.2g, 26....
Embodiment 3
[0136] Embodiment 3 (preparation of compound 1-9)
[0137]
[0138] Step D:
[0139] The intermediate 1b (1.60 g, 3.48 mmol) of Example 1 was added into the reaction flask, methanol (20.0 ml) was added, and stirred at room temperature to dissolve. Sodium methoxide (0.23 g, 4.17 mmol) was added into the reaction system, and the reaction was stirred at room temperature for 1 h. TLC showed the starting material was completely reacted. Add saturated aqueous ammonium chloride solution (15.0ml) to the reaction system, concentrate under reduced pressure, and extract the residue three times with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, suction filter, spin under reduced pressure The steamed crude product was purified by column chromatography to obtain intermediate 1-9d, 1.13g. Yield: 79.66%. MS (ESI+): m / z = 408.2.
[0140] Step E:
[0141] Add compound 1-9d (500mg, 1.22mmol) into the reaction flask, add tetrahyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com