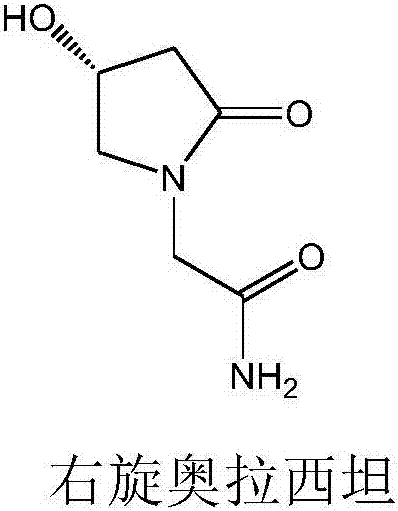
Dextro-oxiracetam crystal form II, and preparation method and application thereof
A crystal form and crystallization technology, which is applied in the field of Dexo-oxiracetam, can solve the problems that there are few researches on the crystal form of Dexo-oxiracetam, and there are no crystal forms and disclosures of Dexo-oxiracetam.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Dissolve 1g of dexoxiracetam (Chongqing Runze Pharmaceutical Co., Ltd.) in 6mL of mixed solvent (DMF 2mL, dichloromethane 4mL) solution, heat to dissolve at 50°C, filter, seal the filtrate, and dissolve at 100~ Stir at a speed of 150r / min for about 15h, and filter again. After filtration, the filtrate is placed in a desiccator to evaporate the solvent to form crystals, collect the crystals, and dry the collected crystals at 30±2°C and a relative humidity of 80-85%. After 5-6h, the crystals of D-oxiracetam were obtained.
Embodiment 2
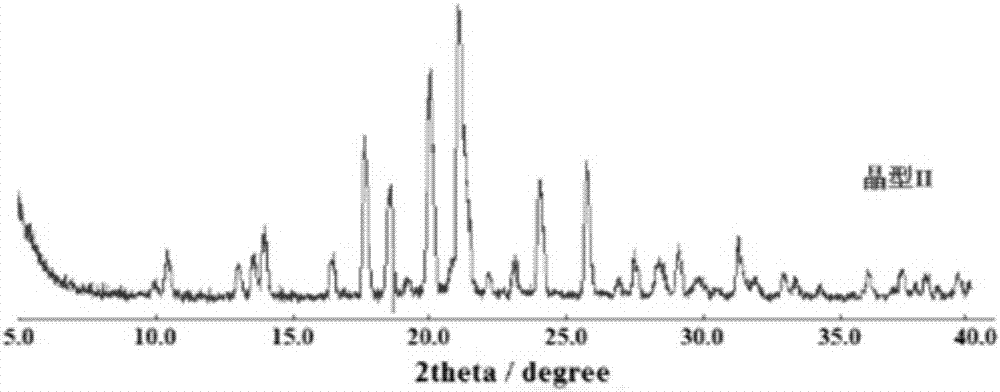
[0041] The dextro-oxiracetam crystal that embodiment 1 obtains is carried out powder diffraction experiment:
[0042] Powder Diffraction Determination (XRPD):
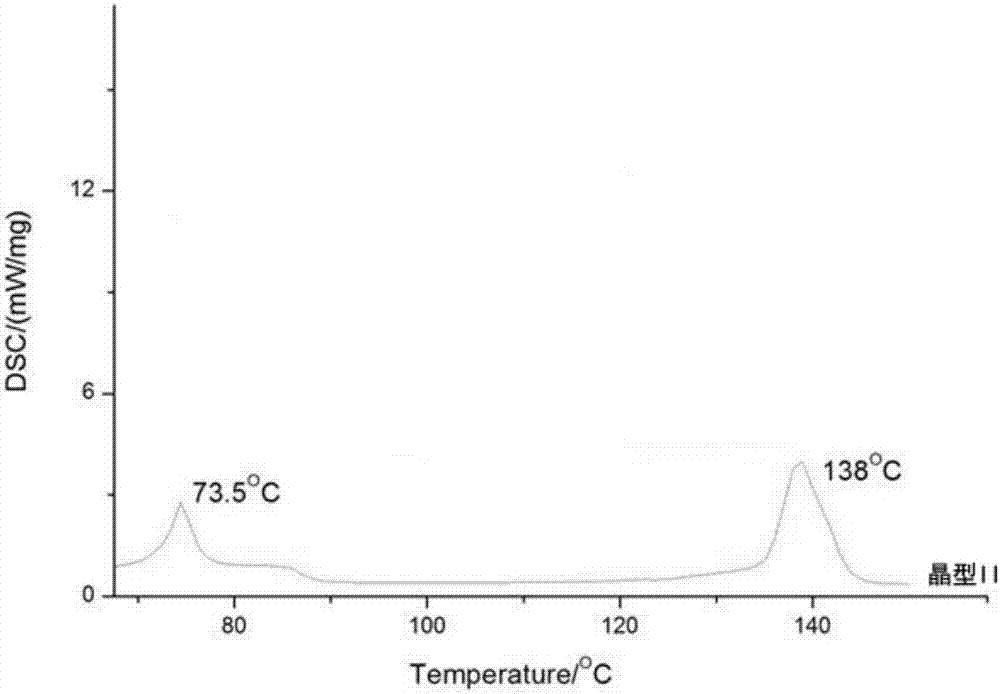
[0043] Test instrument conditions: use Bruker D2 PHASER powder diffractometer to carry out normal temperature test, test conditions are: with Cu Ka ( ) is the light source, the voltage is 30kV, the current is 10mA, the test step size is 0.014°, the scanning speed is 0.1s / step, and the scanning range is 5-40° (2θ). After testing, the D-oxiracetam crystal prepared in Example 1 has diffraction angles 2θ of 10.54±0.2°, 13.76±0.2°, 14.14±0.2°, 16.64±0.2°, 17.76±0.2°, 18.72±0.2° , 20.16±0.2°, 21.20±0.2°, 21.52±0.2°, 23.25±0.2°, 24.17±0.2°, 25.88±0.2°, 27.61±0.2°, 28.57±0.2°, 29.24±0.2°, 31.40±0.2° There are diffraction peaks, for the sake of convenience, this crystal is called "dextro-oxiracetam crystal form II"; its powder diffraction pattern is shown in figure 1 . Depend on figure 1 It can be seen from the analytic...
Embodiment 3
[0049] Dissolve 1g of Dexoxiracetam in 5mL of mixed solvent (DMF 1mL, ethyl acetate 4mL), heat to dissolve at 35°C, filter, cover and seal the filtrate, and stir at a speed of about 150r / min for about 12h , filtered again, and after filtration, the filtrate was allowed to stand in a desiccator to evaporate the solvent to form crystals, and the crystals were collected, and the collected crystals were dried at 30±2° C. and a relative humidity of 75-80% for 4-5 hours to obtain crystals; According to the method identification in Example 2, it is Dexoxiracetam crystal form II.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition peak temperature | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com