A kind of medicinal pellet core and preparation method thereof
A technology for pellet cores and pellets, applied in the field of medicinal pellet cores and their preparation, can solve the problems of particle size uniformity, roundness and surface finish not reaching a satisfactory level, and unstable product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
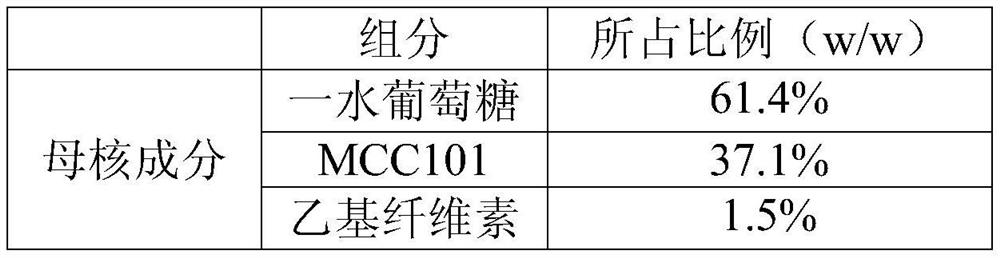
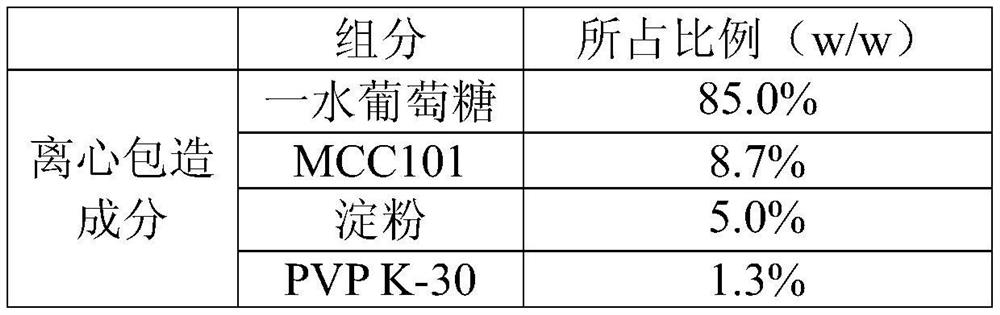
[0023] Embodiment 2. The anhydrous glucose pellet core, wherein the dosage ratio of the mother core component and the centrifuge encapsulation component is 1:2.
[0024]
[0025]
[0026] The preparation method is:
[0027] a. Crush and sieve each component, weigh each component according to the prescription amount, dissolve PVP K-30 in water to make an aqueous binder solution, and place the anhydrous glucose and MCC101 in the mother core component in a wet granulator After mixing evenly, add the binder aqueous solution to prepare the mother core.
[0028] b. Turn on the centrifugal packaging machine, put the prepared mother core into the centrifugal packaging machine, adjust the rotating speed, and use the anhydrous glucose and starch in the centrifugal packaging composition to supply powder while spraying the binder solution. The mother core is layered and powdered, and finally the prepared pellets are sieved.
Embodiment 3
[0029] Embodiment 3. The anhydrous glucose pellet core, wherein the dosage ratio of the mother core component and the centrifuge encapsulation component is 1:2.5.
[0030]
[0031]
[0032]
[0033] The preparation method is:
[0034] a. Crush and sieve each component, weigh each component according to the prescription amount, dissolve PVP K-30 in water to make an aqueous binder solution, and place the anhydrous glucose and MCC101 in the mother core component in a wet granulator After mixing evenly, add the binder aqueous solution to prepare the mother core.
[0035] b. Turn on the centrifugal packaging machine, put the prepared mother core into the centrifugal packaging machine, adjust the rotating speed, and use the anhydrous glucose and MCC101 in the centrifugal packaging composition to supply powder while spraying the binder solution. The mother core is layered and powdered, and finally the prepared pellets are sieved.
Embodiment 4
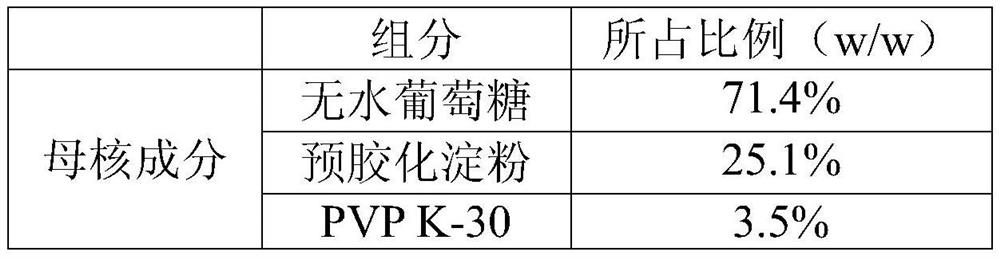
[0036] Embodiment 4. Glucose monohydrate pellet core, wherein the dosage ratio of mother core component and centrifuge encapsulation component is 1:2.5.
[0037]
[0038]
[0039] The preparation method is:
[0040] a. Crush and sieve each component, weigh each component according to the prescription amount, dissolve PVP K-30 in water to make an aqueous binder solution, and place glucose monohydrate and MCC101 in the mother core component in a wet granulator After mixing evenly, add the binder aqueous solution to prepare the mother core.
[0041]b. Turn on the centrifugal packaging machine, put the prepared mother core into the centrifugal packaging machine, adjust the rotating speed, and use the glucose monohydrate and MCC101 in the centrifugal packaging composition to supply powder while spraying the binder solution. The mother core is layered and powdered, and finally the prepared pellets are sieved.
[0042] 2. Determination of traits or properties:
[0043] 1) Ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com