Non-noble metal oxygen evolution reaction catalyst and preparation method thereof
An oxygen evolution reaction, non-precious metal technology, used in oxygen preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as poor stability and high cost, improve kinetic process, reduce energy barrier and enhance the effect of electron transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
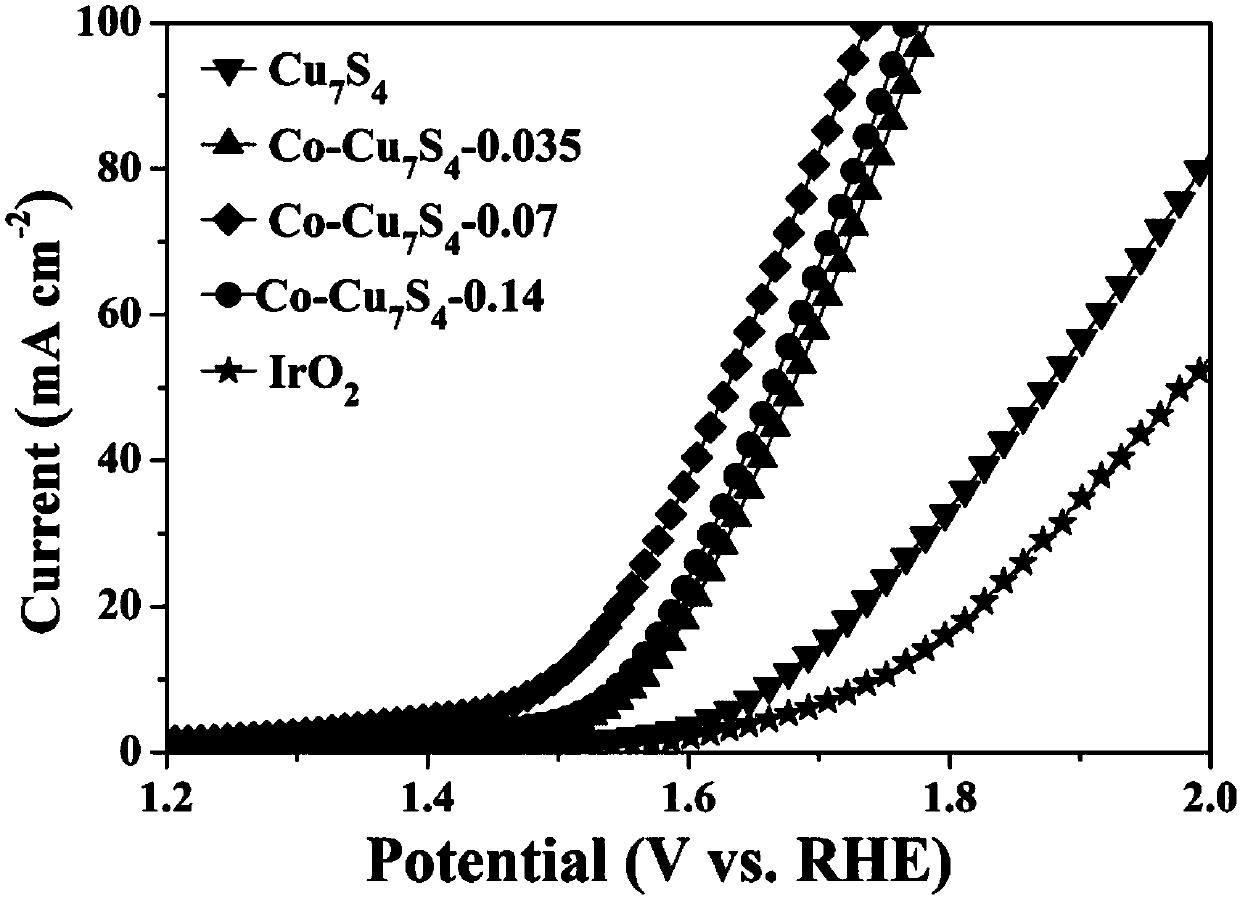
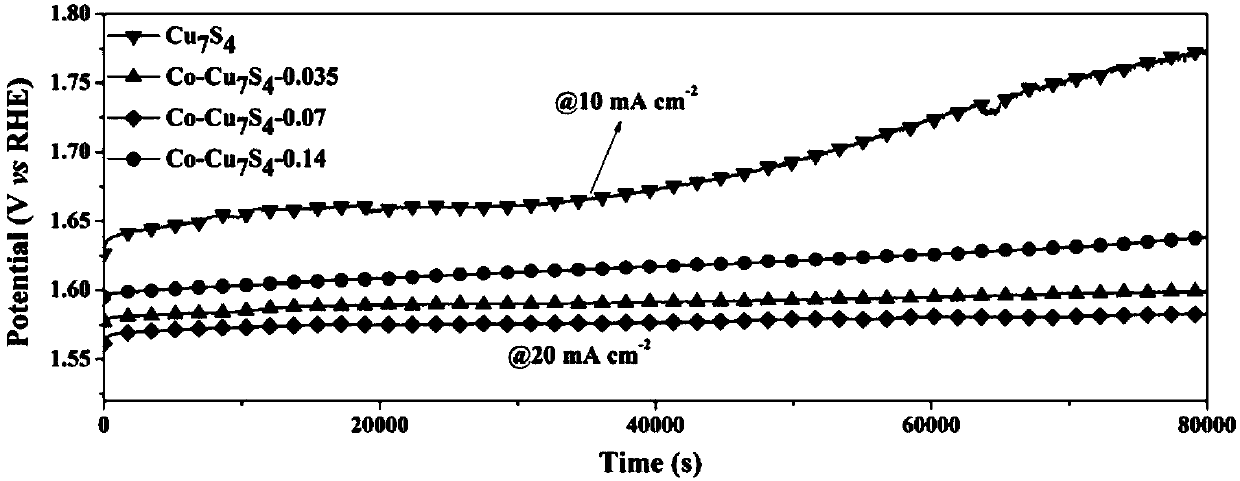
[0022] This embodiment provides a non-noble metal oxygen evolution reaction catalyst, that is, cobalt-doped Cu 7 S 4 , the doping amount of cobalt is 7% of the total molar weight of Cu and Co in this catalyst (catalyst is recorded as Co-Cu 7 S 4 -0.07);
[0023] Concrete preparation method comprises the following steps:
[0024] (a) 193.14mg of cuprous thiocyanate and 40.7mg of cobalt acetylacetonate are mixed and poured into a 250ml flask;
[0025] (b) Add 100ml of oleic acid to the flask and stir evenly, then continuously feed argon into the flask for 20 minutes, first raise the temperature to 140°C, and continue for 30 minutes; continue to heat up to 240°C, and react for 30 minutes;
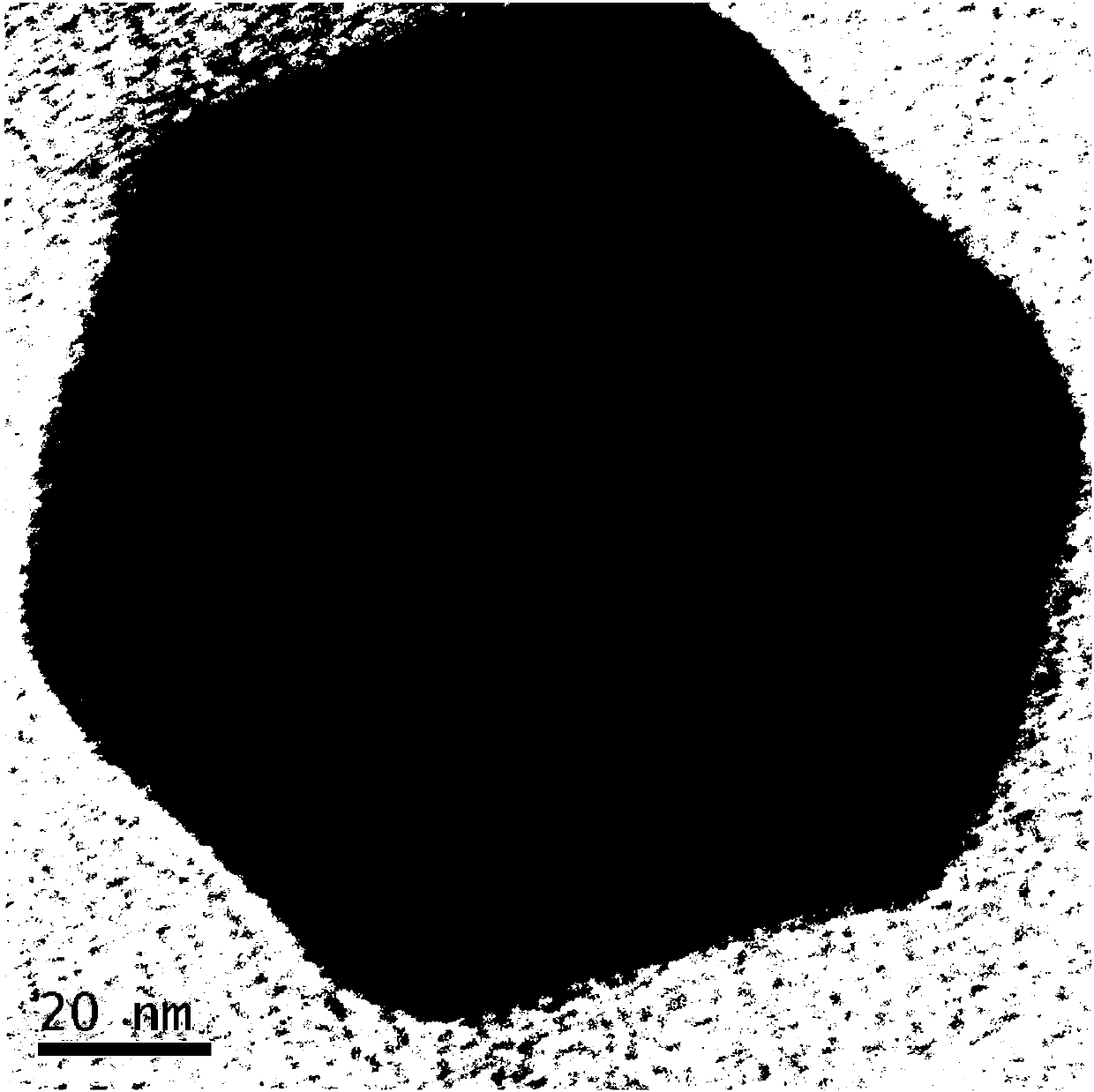
[0026] (c) Transfer the flask into ice water to cool down quickly, take out the reaction product and add it to alcohol for centrifugal cleaning; then transfer it to the refrigerator and freeze it into a solid state, which can be obtained after freeze-drying, and its appearance is as follow...
Embodiment 2
[0028] This embodiment provides a non-noble metal oxygen evolution reaction catalyst (the catalyst is denoted as Co-Cu 7 S 4 -0.035), Cu 7 S 4 Cobalt is doped in the crystal, and the doping amount of cobalt is 3.5% of the total molar weight of Cu and Co in this catalyst; Its preparation method is basically the same as that in Example 1, and difference is: in step (a), will 193.14mg of cuprous thiocyanate and 20.4mg of cobalt acetylacetonate were mixed and poured into a 250ml flask.
Embodiment 3
[0030] This embodiment provides a non-noble metal oxygen evolution reaction catalyst (the catalyst is denoted as Co-Cu 7 S 4 -0.14), Cu 7 S 4 Cobalt is doped in the crystal, and the doping amount of cobalt is 14% of the total molar weight of Cu and Co in this catalyst, and its preparation method is basically the same as that in Example 1, and difference is: in step (a), will 193.14mg of cuprous thiocyanate and 81.4mg of cobalt acetylacetonate were mixed and poured into a 250ml flask.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com