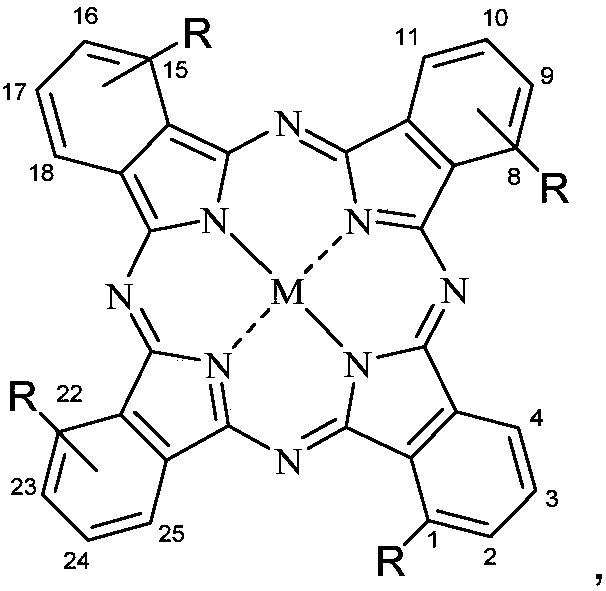
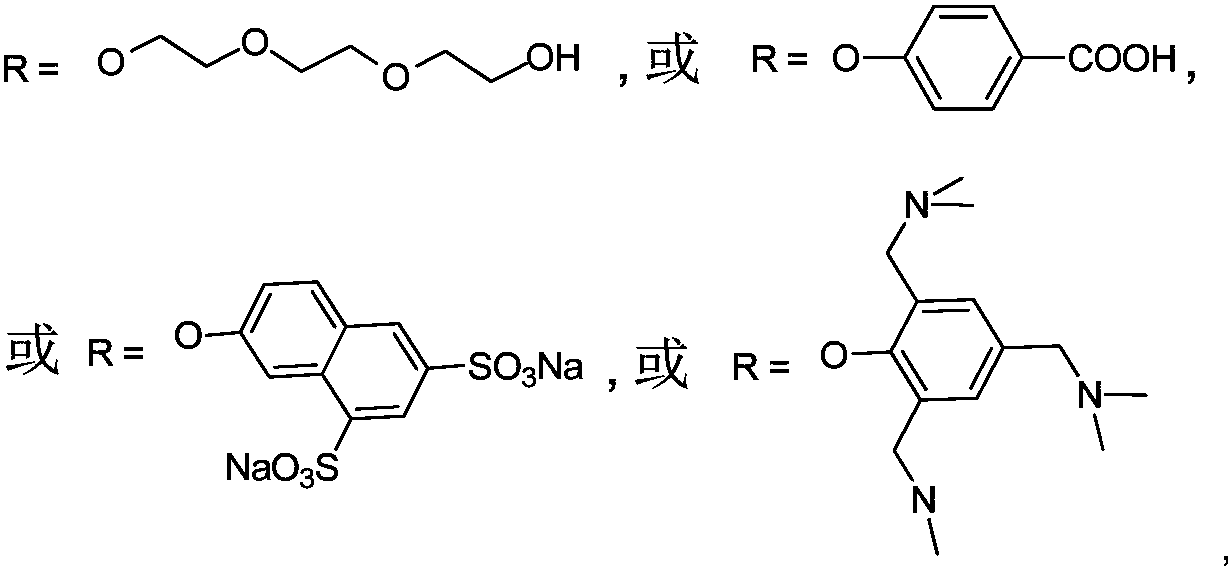
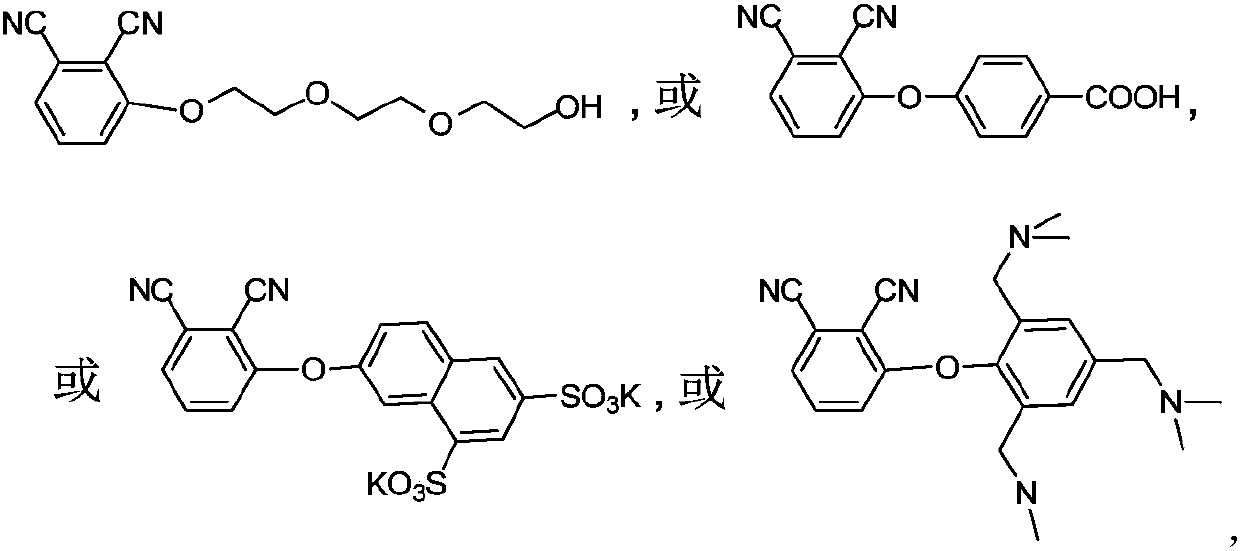
Metal phthalocyanine and application thereof in fields of photothermal materials and photothermal therapy
A metal phthalocyanine and metal technology, which is applied in the field of photothermal agents and photothermal therapy drugs, can solve the problems of limited and unseen photothermal therapy application research, and achieve the goal of broadening applications, good photothermal effect, and high photothermal effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of tetracarboxyl substituted cobalt phthalocyanine complexes with the following structure
[0053] in:
[0054] (1) prepare the phthalonitrile derivative of following formula of structure:
[0055]
[0056] With 3-nitrophthalonitrile (20mmol) and p-hydroxybenzoic acid (20-70mmol, preferably 60mmol) as reactants, dimethyl sulfoxide (40-200mL, preferably 140mL) as solvent, in potassium carbonate ( In the presence of 30-90 mmol, preferably 80 mmol) and nitrogen protection, the reaction was stirred at room temperature to 60°C (preferably 60°C) for 24-72 hours, and monitored by thin-layer chromatography. After the reaction, the reaction solution was poured into ice water, and part of the pale yellow precipitate was precipitated, filtered, and the filter residue was collected, and the filtrate was washed with CHCl 3 Extraction, and then back extract the extract with water, CHCl 3 The extract was rotary evaporated and dried under vacuum at room temperature ...
Embodiment 2
[0066] Synthesis of tetracarboxyl substituted nickel phthalocyanine complexes with the following structure
[0067] in:
[0068] Preparation of tetrasubstituted carboxyl nickel phthalocyanine complex: with the phthalonitrile derivative (2mmol) described in the above-mentioned Example 1 (1) as the reactant, n-amyl alcohol (20-34mL, preferably 30mL) as the solvent, add Nickel chloride (1~4mmol, preferably 2mmol), with 1,8-diazabicyclo[5.4.0]undec-7-ene (0.4~1.2mL, preferably 0.6mL) as catalyst, 130~150 Stir the reaction at ℃ for 12-48 hours, monitor the end point of the reaction by thin-layer chromatography, and generate the corresponding nickel phthalocyanine complex. After the reaction, evaporate to dryness, dissolve with a small amount of DMF, pass through a silica gel column, remove yellow impurities with ethyl acetate, collect the target band with DMF / acetic acid (20 / 1), and evaporate to dryness. Add a small amount of DMF to dissolve, add water to precipitate, and dry...
Embodiment 3
[0071] Using manganese chloride instead of copper chloride in Example 1, the corresponding tetracarboxy substituted manganese phthalocyanine complex can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com