Preparation method and application of biphenyl aromatic compound-substituted nickel phosphosulfonate complex
A technology of aromatic compounds and nickel complexes, applied in the direction of nickel organic compounds, etc., can solve the problems of low insertion ratio, low copolymer molecular weight, low activity, etc., and achieve the effect of high insertion ratio, high copolymer molecular weight, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
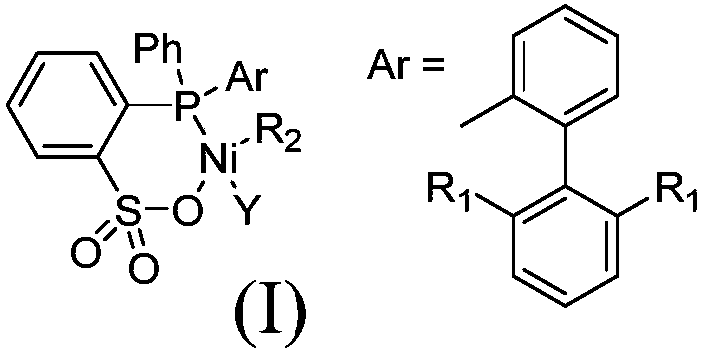
[0026] Synthesis of 2-((2-(2,6-dioxoisopropylphenyl)phenyl)(phenyl)phosphino)benzenesulfonic acid
[0027]
[0028] Under the protection of nitrogen at 0°C, n-butyllithium (concentration: 2.5 mol per liter, 8 ml, 20 mmol) was slowly added dropwise to 25 ml of tetrahydrofuran mixed solution dissolved with benzenesulfonic acid (1.58 g, 10 mmol) , reacted for 1 hour to obtain a reaction mixture. Then, the reacted mixture was added into 25 ml of tetrahydrofuran mixed solution dissolved with phenylphosphine dichloride (1.35 ml, 10 mmol) at 0° C., and reacted at room temperature for 2 hours to obtain mixed solution A.
[0029] 2-(2-Bromophenyl)-1,3-dioxocumene (3.49 g, 10 mmol) was dissolved in 20 ml of tetrahydrofuran under nitrogen protection and cooled to minus 78 ° C, and then n-butyl Lithium (concentration: 2.5 mol per liter, 4 ml, 10 mmol) was slowly added thereto, and reacted at minus 78° C. for 1 hour. The obtained lithium salt was added to the mixed solution A at minus...
Embodiment 2
[0032] Synthesis of 2-((2-(2,6-difluorophenyl)phenyl)(phenyl)phosphino)benzenesulfonic acid
[0033]
[0034]Under the protection of nitrogen at 0°C, n-butyllithium (concentration: 2.5 mol per liter, 8 ml, 20 mmol) was slowly added dropwise to 25 ml of tetrahydrofuran mixed solution dissolved with benzenesulfonic acid (1.58 g, 10 mmol) , reacted for 1 hour to obtain a reaction mixture. Then, the reacted mixture was added into 25 ml of tetrahydrofuran mixed solution dissolved with phenylphosphine dichloride (1.35 ml, 10 mmol) at 0° C., and reacted at room temperature for 2 hours to obtain mixed solution A.
[0035] 2-(2-Bromophenyl)-1,3-difluorobenzene (2.69 g, 10 mmol) was dissolved in 20 ml of tetrahydrofuran under the protection of nitrogen and the temperature was lowered to minus 78 ° C, then n-butyllithium (concentration 2.5 moles per liter, 4 milliliters, 10 mmoles) were slowly added therein, and reacted for 1 hour at minus 78°C. The obtained lithium salt was added t...
Embodiment 3
[0038] Synthesis of Nickel Complexes of 2-((2-(2,6-Dioxyisopropylphenyl)phenyl)(phenyl)phosphino)benzenesulfonate
[0039]
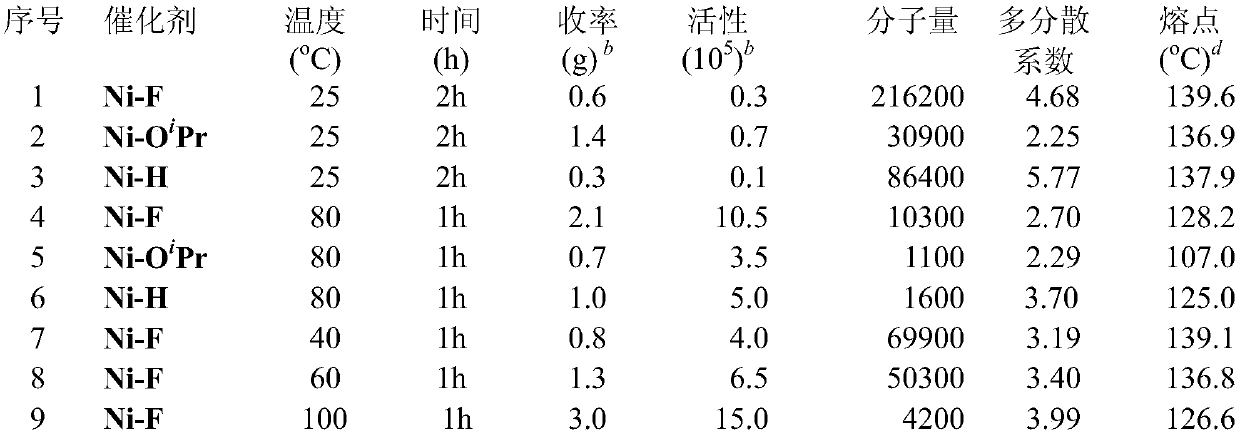
[0040] Catalyst (Ni-O i Pr)
[0041] Take a 100 ml Schlenk bottle and add 2-(2,6-dioxoisopropylphenyl)phenyl)(phenyl)phosphino)benzenesulfonic acid (534 mg, 1 mmol), sodium hydride (29 mg, 1.2 mmol) and 20 mL THF. After stirring at room temperature for 12 h, the resulting yellow solution was passed over celite and dried on a vacuum line. It was then dissolved in 20 mL of dichloromethane and added (PPh 3 ) 2 NiPhCl (694 mg, 1 mmol) was placed in a glove box and stirred at room temperature with a magnetic stirrer for 12 hours, and the resulting mixture was filtered through celite. The filtrate was vacuum-pumped to dry the solvent, and dried to obtain a yellow powder. Wash the powder with a 2:1 mixed solvent of n-hexane and toluene. A yellow solid (670 mg, 72%) was obtained.
[0042] 1H NMR (CDCl3, 400MHz): δ7.82-7.78(m, 2H), 7.69-7.66(m, 2H), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com