Highly-sensitive method for determining residual quantity of piperidine in bulk drug
A technology of raw materials and detection methods, which is applied in the field of high-sensitivity detection, can solve the problems of not meeting the limit requirements and poor sensitivity, and achieve the effects of increased sensitivity, accurate and reliable results, and simple and fast methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the qualitative of piperidine derivative
[0048] Instruments and Conditions:
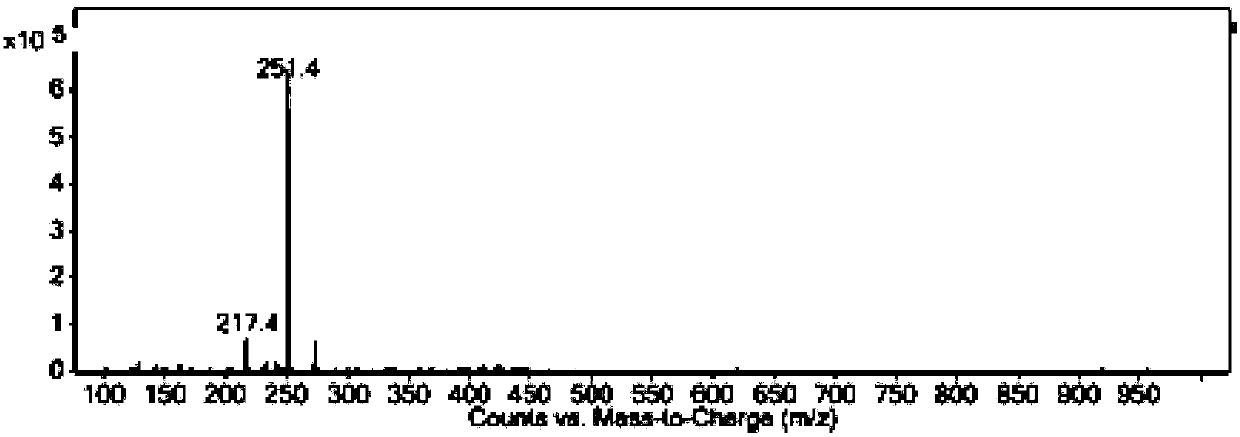
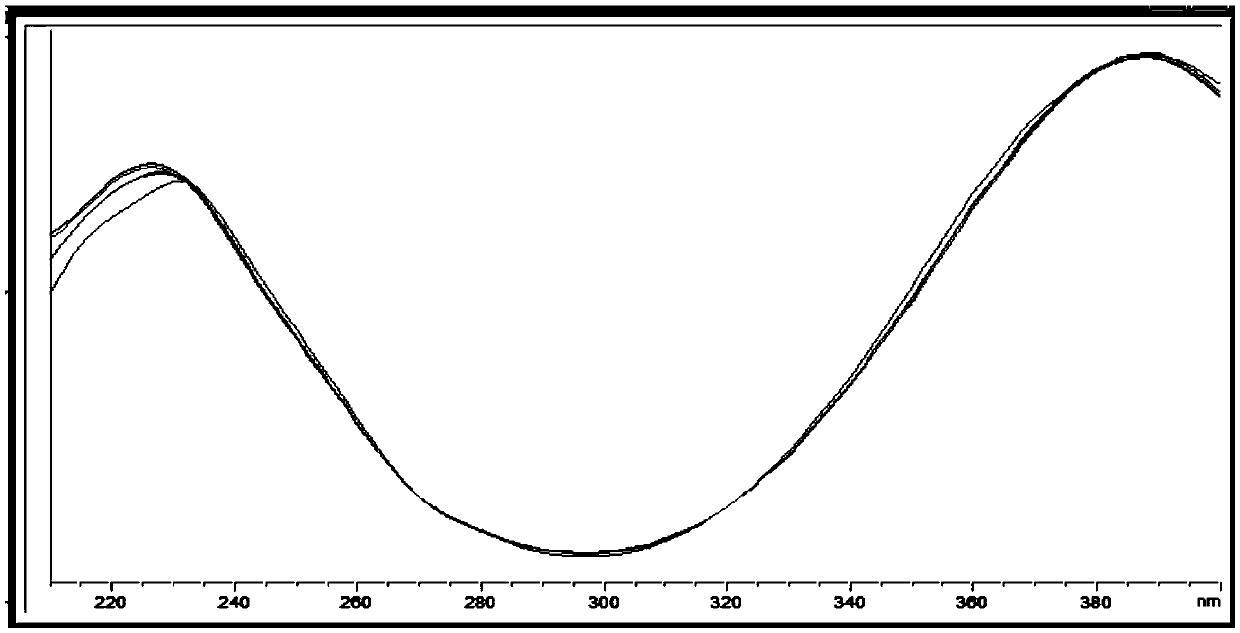
[0049] Agilent1290 liquid chromatograph, pump model is G4220A, ultraviolet detector model G4212A, electrospray ionization detector model is G6460B and Chemstation processing software, chromatographic column: phenomenonex C18 (250*4.6mm, 5μm), ultraviolet detection wavelength It is 388nm, and the mass spectrum parameters are: Fragment: 135; Cell Accelerator: 7; Positive.
[0050]Mobile phase: acetonitrile-water (65:35, v / v), flow rate 1ml / min, injection volume 10μl.
[0051] Experimental steps:
[0052] Preparation of piperidine reference substance stock solution: Take an appropriate amount of reference substance (AR, batch number: J01010, Beijing Chemical Plant), dissolve it in DMSO, and prepare a piperidine reference substance stock solution with a final concentration of 0.1 mg / ml.
[0053] Preparation of 2,4-dinitrofluorobenzene stock solution: Take an appropriate amount o...
Embodiment 2
[0057] Example 2 Derivatization reaction time investigation
[0058] Instruments and Conditions
[0059] Agilent1260 liquid chromatograph, the model of pump is G1311A, the ultraviolet detector model G1315D, and Chemstation processing software, chromatographic column: phenomenonex C18 (250*4.6mm, 5 μ m), ultraviolet detection wavelength is 388nm, mobile phase: acetonitrile-water ( 65:35, v / v), flow rate 1ml / min, injection volume 10μl.
[0060] experiment procedure:
[0061] Preparation of piperidine reference substance stock solution: Take an appropriate amount of reference substance and dissolve it in DMSO to a final concentration of 0.1 mg / ml.
[0062] Preparation of 2,4-dinitrofluorobenzene stock solution: Take an appropriate amount of sample and dissolve it in DMSO to a final concentration of 4 mg / ml.
[0063] Derivation reaction: Take 1ml of piperidine reference substance, put it into a 100ml volumetric flask, add 1ml of 2,4-dinitrofluorobenzene stock solution precisely...
Embodiment 3
[0066] Embodiment 3: the determination of liquid chromatography condition:
[0067] Analyze according to different liquid chromatography conditions in Table 1
[0068] Table 1:
[0069]
[0070] .
[0071] System sample preparation conditions are shown in Table 2:
[0072] Table 2
[0073]
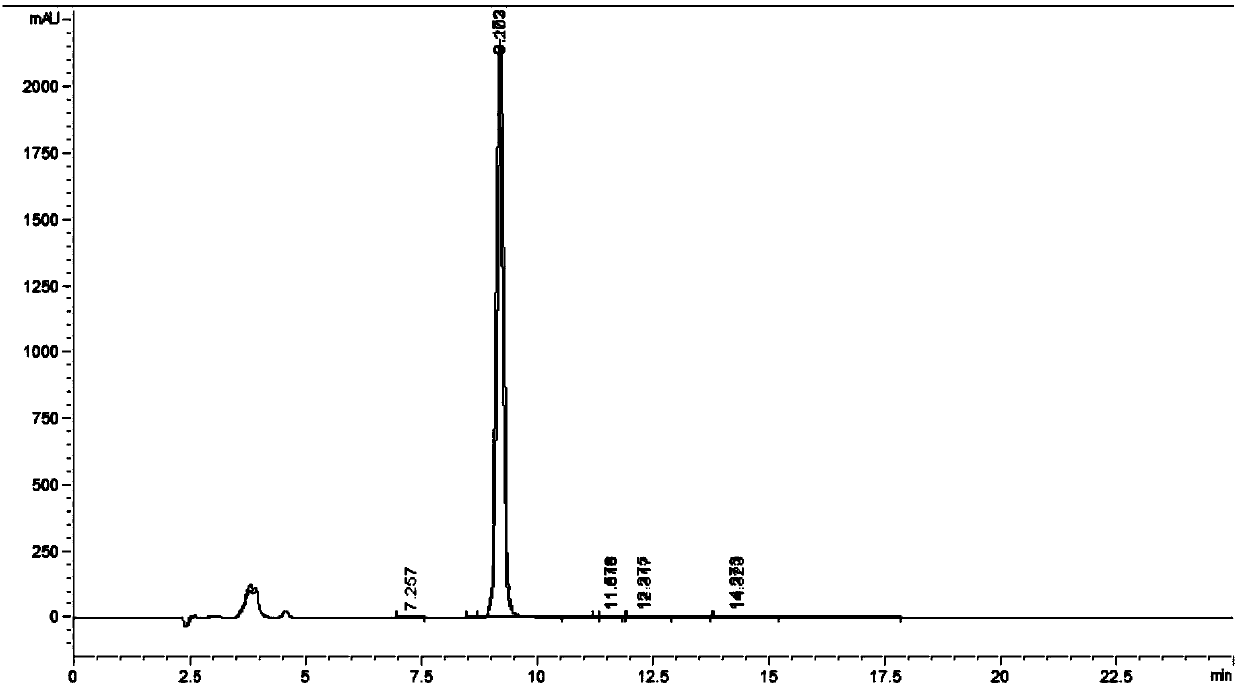
[0074] see results Figure 4 and Figure 5 .
[0075] Test conclusion: meet the chromatographic conditions of above-mentioned condition 1 and condition 2, piperidine derivatives all can go out peak normally, and retention time changes to some extent, but does not affect normal determination, considers that piperidine derivatives peak shape is best (as peak width minimum, high resolution), the optimum flow rate is 1ml / min, the optimum column temperature is 30°C, and the optimum injection volume is 10μl.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com