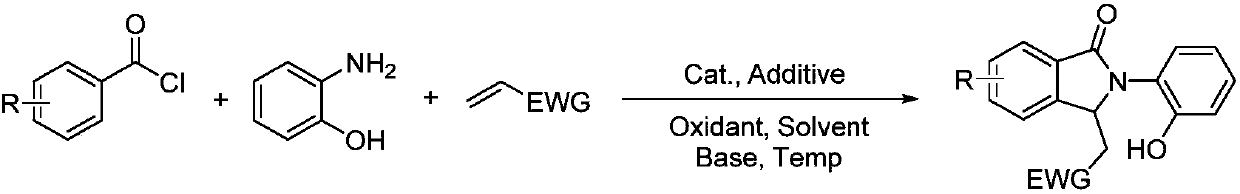
Synthesis method of polysubstituted isoindoline derivative
A technology of isoindolinone and a synthesis method, applied in the field of organic synthesis, can solve the problems of complicated steps, difficult source of raw materials, high price, etc., and achieves the effects of good reaction universality, convenient source of raw materials, and good promotion prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
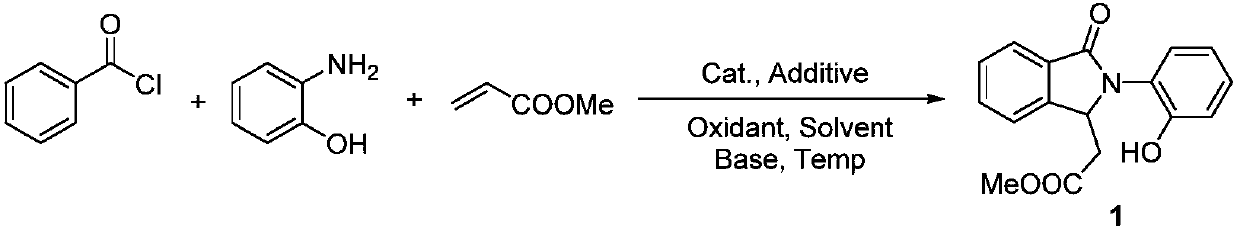
[0020] Synthesis of Isoindolinone Derivative 1
[0021]
[0022] Benzoyl chloride (5mmol), o-aminophenol (10mmol), methyl acrylate (5mmol), [Rh(cod)Cl] 2 (0.05mmol), AgSbF 6 (0.1mmol), copper acetate (2mmol), sodium hydroxide (10mmol) and acetonitrile (5mL) were added to the reaction tube, mixed thoroughly and heated to 60 ° C and kept for 24 hours; after the reaction, the resulting mixture was poured into ice water, extracted with ethyl acetate, fully washed with deionized water, dried, and distilled under reduced pressure to obtain a crude product. Through TLC spot plate analysis, there were no obvious by-products except the remaining raw materials, and almost only one new substance was produced. Chromatography obtained 1.04g of pure product, with a yield of 70% (the yield is based on the molar weight of aroyl chloride in the raw material, the same below), and was detected as the target product by mass spectrometry and carbon spectrometry:
[0023] 1 H NMR (300MHz, CDC...
Embodiment 2
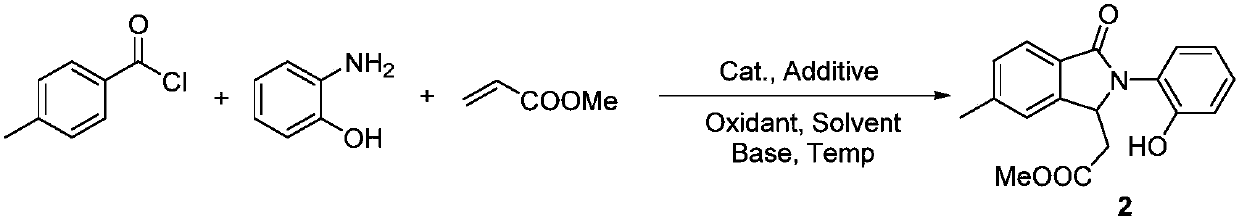
[0025] Synthesis of Isoindolinone Derivative 2
[0026]
[0027] P-toluoyl chloride (5mmol), o-aminophenol (10mmol), methyl acrylate (15mmol), [Cp*RhCl 2 ] 2 (0.25mmol), AgSbF 6 (2mmol), copper acetate (5mmol), potassium carbonate (10mmol) and acetonitrile (50mL) were added to the reaction tube, after mixing thoroughly, the temperature was raised to 120°C and kept for 6 hours; after the reaction, the resulting mixture was poured into ice In water, extracted with ethyl acetate, fully washed with deionized water, dried, and distilled under reduced pressure to obtain a crude product. Through TLC spot plate analysis, there were no obvious by-products except the remaining raw materials, and almost only one new substance was formed. After column chromatography Obtain 1.48g of pure product, the yield is 95%, and it is the target product detected by mass spectrometry and carbon spectrometry:
[0028] 1 H NMR (300MHz, DMSO-d6) δ9.78(s, 1H), 7.64(d, J=7.7Hz, 1H), 7.44(s, 1H), 7.3...
Embodiment 3
[0030] Synthesis of Isoindolinone Derivative 3
[0031]
[0032] P-chlorobenzoyl chloride (5mmol), o-aminophenol (10mmol), methyl acrylate (10mmol), [RuCl 2 (p-Cymene)] 2 (0.1mmol), AgBF 4 (0.4mmol), copper acetate (3mmol), triethylamine (10mmol)
[0033] -3-
[0034] and acetonitrile (10mL) were added to the reaction tube, mixed thoroughly, heated to 100°C and kept for 12 hours of reaction; after the reaction, the resulting mixture was poured into ice water, extracted with ethyl acetate, fully washed with deionized water, and dried. The crude product was obtained by distillation under reduced pressure. Through TLC spot plate analysis, there were no obvious other by-products except the remaining raw materials, and almost only a new substance was generated. The pure product was 1.36g through column chromatography, and the yield was 82%. After mass spectrometry, carbon Spectral detection for the target product:
[0035] 1 H NMR (300MHz, CDCl 3 )δ8.09(s,1H),7.86(d,J=8.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com