Novel protein expression system U2-OS and application thereof
A protein expression and U2-OS technology, applied in the field of bioengineering, can solve the problems of low yield and low activity, and achieve the effects of simple preparation method, efficient post-translational modification system and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Expression of hBMP-7 in human osteosarcoma U2-OS cells
[0051] The self-signal peptide gene sequence of hBMP-7 described in this implementation:
[0052] SEQ ID NO:1
[0053] ATGCACGTGCGCTCACTGCGAGCTGCGGCGCCGCACAGCTTCGTGGCGCTCTGGGCACCCCTGTTCCTGCTGCGCTCCGCCCTGGCC
[0054] The self-signal peptide amino acid sequence of hBMP-7 described in this implementation
[0055] SEQ ID NO:2
[0056] MHVRSLRAAAAPHSFVALWAPLFLLRSALA
[0057] The hBMP-7 mature peptide gene sequence described in this implementation
[0058] SEQ ID NO:3
[0059] TCCACGGGGAGCAAACAGCGCAGCCAGAACCGCTCCAAGACGCCCAAGAACCAGGAAGCCCTGCGGATGGCCAACGTGGCAGAGAACAGCAGCAGCGACCAGAGGCAGGCCTGTAAGAAGCACGAGCTGTATGTCAGCTTCCGAGACCTGGGCTGGCAGGACTGGATCATCGCGCCTGAAGGCTACGCCGCCTACTACTGTGAGGGGGAGTGTGCCTTCCCTCTGAACTCCTACATGAACGCCACCAACCACGCCATCGTGCAGACGCTGGTCCACTTCATCAACCCGGAAACGGTGCCCAAGCCCTGCTGTGCGCCCACGCAGCTCAATGCCATCTCCGTCCTCTACTTCGATGACAGCTCCAACGTCATCCTGAAGAAATACAGAAACATGGTGGTCCGGGCCTGTGGCTGCCAC
[0060] The hBMP...
Embodiment 2
[0095] Example 2 Expression of hBMP-7 recombinant adenovirus vector in human osteosarcoma U2-OS cells
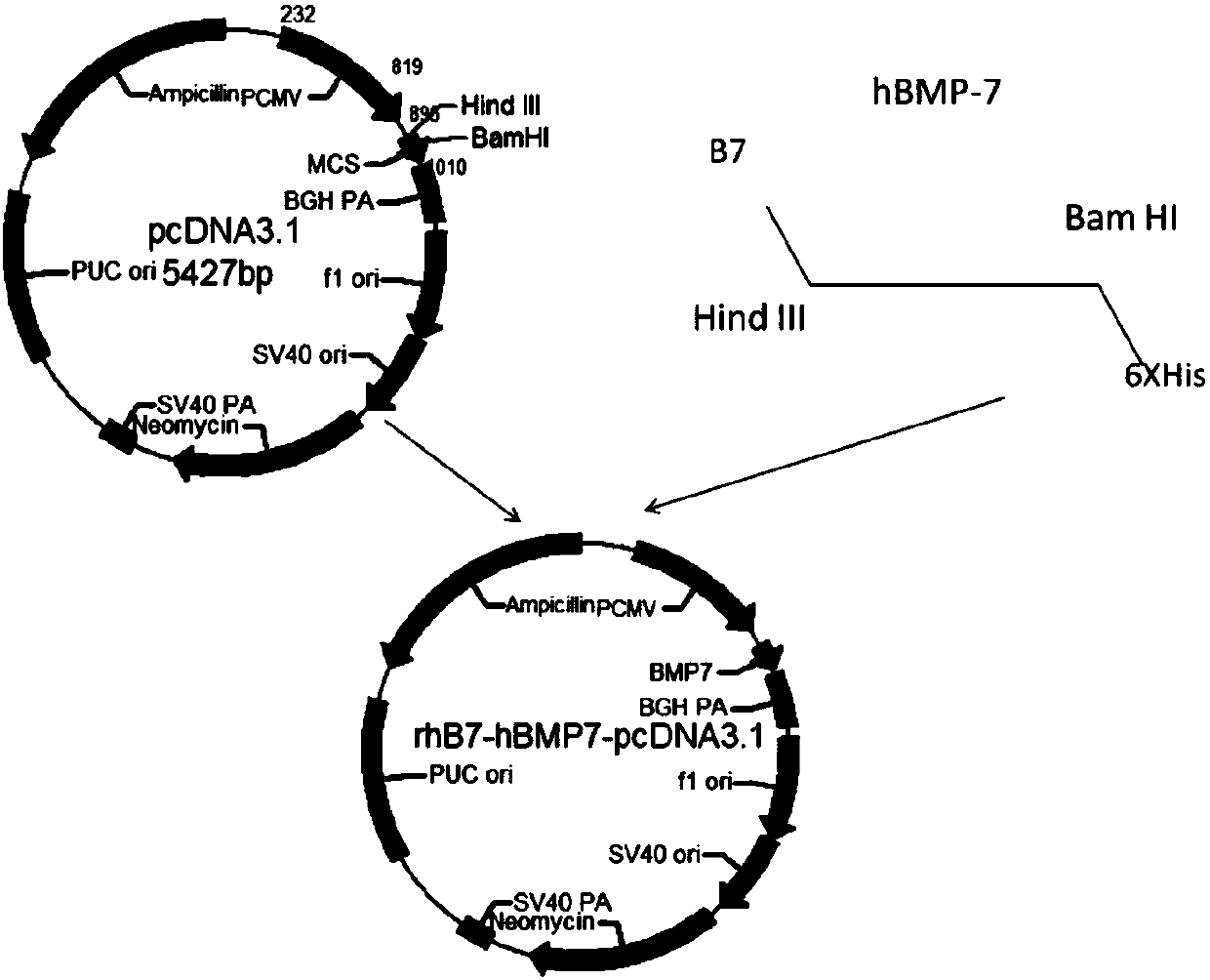
[0096] Add its own signal peptide gene sequence at the 5' end of the hBMP-7 mature peptide gene sequence, and add a Not I restriction site at the 5' end of the signal peptide gene sequence, and add 6XHis tag and Hind at the 3' end of the hBMP-7 mature peptide gene sequence III restriction site. The designed human hBMP-7 gene (rhB7-hBMP-7) sequence was synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd. The synthesized gene sequence was digested with Not I and Hind III and inserted into the pShuttle-CMV shuttle vector to construct the recombinant adenovirus shuttle plasmid pShuttle-hBMP7. Eco RI digested pShuttle-hBMP7, and combined with pAdEasy TM DNA electroporation co-transformed Bj5183 competent cells, extracted the recombinant AdBMP7 plasmid, digested with Pac I to linearize the recombinant plasmid AdBMP7, transfected Ad293 cells for virus packaging and amplificati...
Embodiment 3
[0097] Example 3 Expression of hBMP-2 in human osteosarcoma U2-OS cells
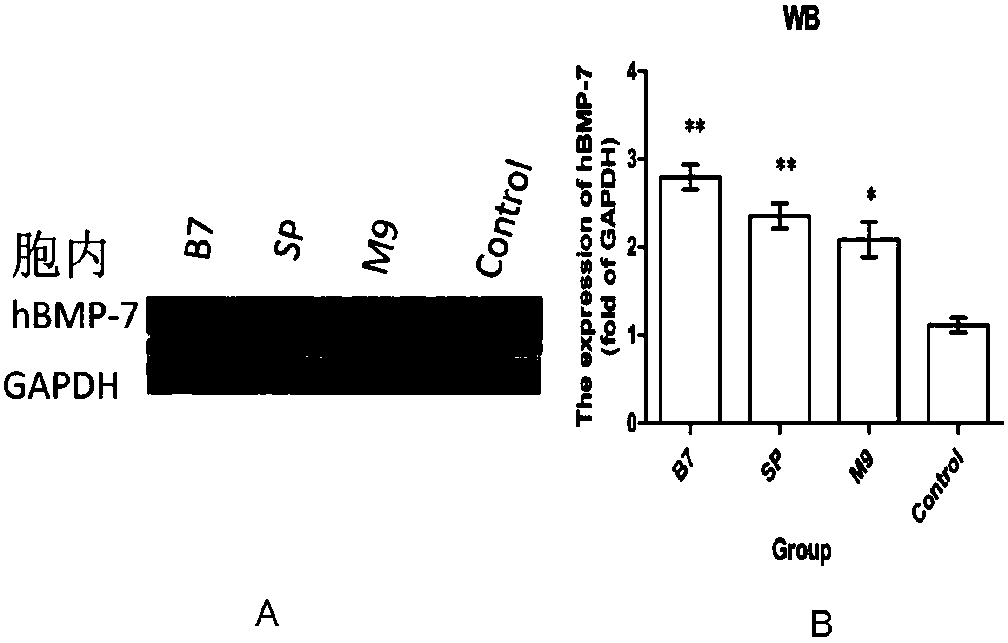
[0098] Replace the amino acid sequence of hBMP-7 with the amino acid sequence of hBMP-2, add the signal peptide of hBMP-7 to the amino terminal of hBMP-2, and add a Hind III restriction site at the 5' end of the signal peptide gene sequence, add 6ΧHis tag and Bam HI restriction site. Both the recombinant expression vector rhB7-hBMP-2-pcDNA3.1 and the recombinant adenovirus expression vector AdBMP2 constructed separately can be expressed in human osteosarcoma U2-OS cells, and the expression products are glycosylated and have high ALP activity Recombinant protein activity obtained from mammalian or microbial protein expression systems.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com