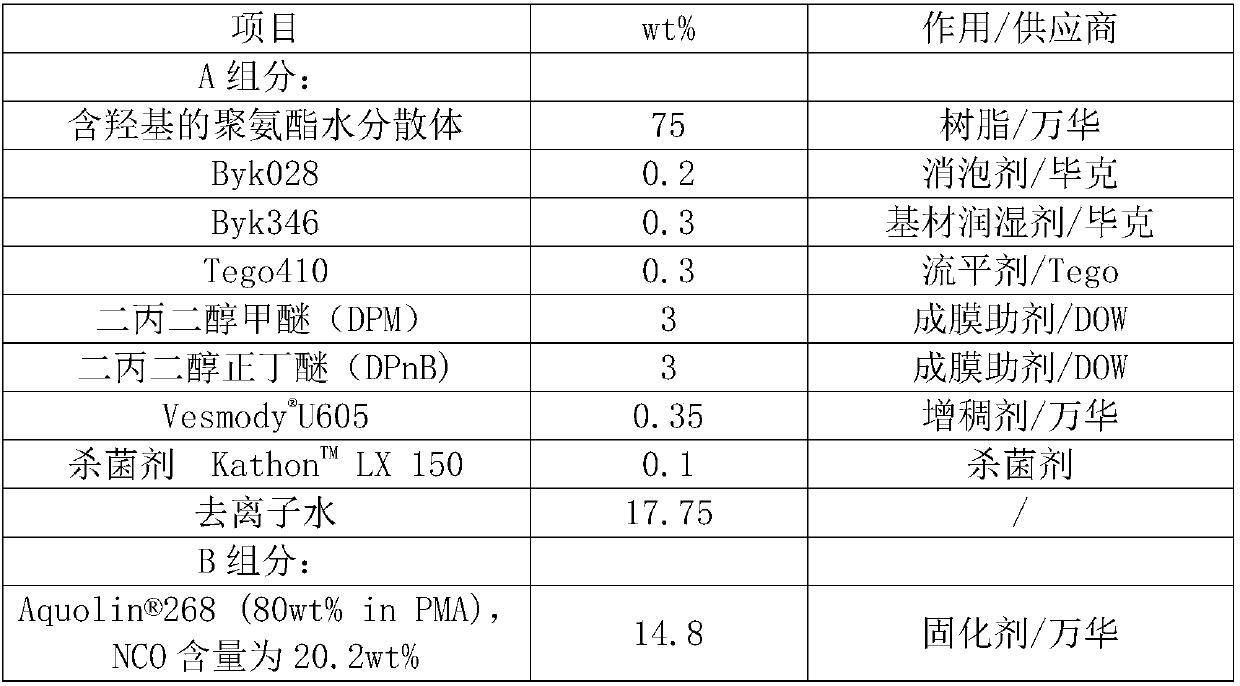
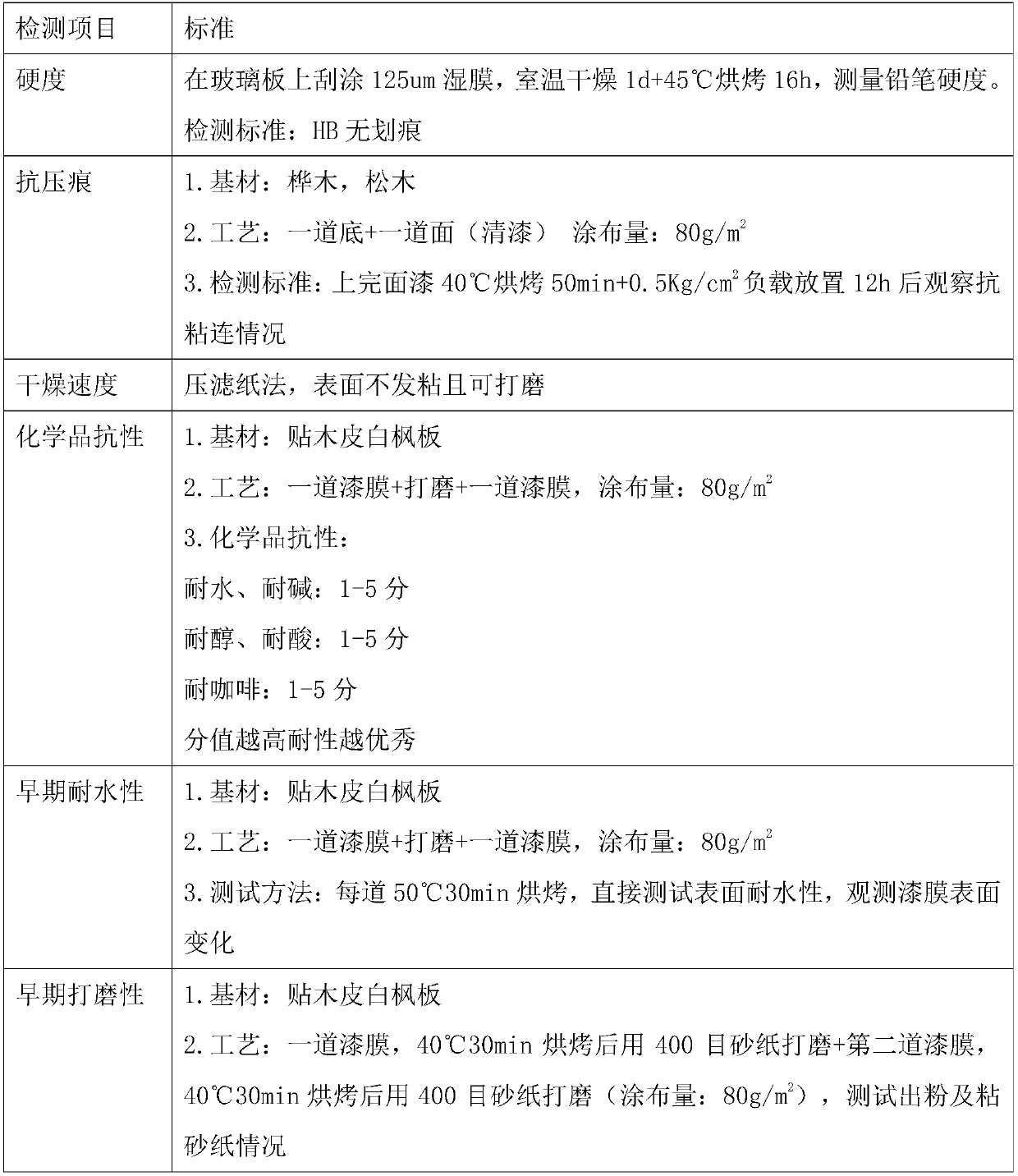
Polyurethane aqueous dispersion containing hydroxy group as well as preparation method and application of polyurethane aqueous dispersion
A polyurethane water dispersion and polyurethane technology, applied in polyurea/polyurethane coatings, coatings, primers, etc., can solve problems such as low double bond content, low glass transition temperature, and poor paint film polishability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Add 80g of dehydrated vegetable oil modified polyol 1, 1.8g of dehydrated Ymer N-120, 50g of WANOL R2307, 93g of TDI, 28.05g of HMDI, and 38g of acetone into a 1L four-neck round-bottomed flask equipped with a nitrogen inlet and outlet, The mixture was stirred at 80-90°C until NCO reached 9.28 wt%. Cool down and add 16g of 1,6-hexanediol, 8.0g of dimethylolpropionic acid and 50g of acetone, raise the temperature to 70°C and continue the reaction until the NCO reaches 2.92wt%. Lower the temperature and add 33.8g of trimethylolpropane and 96g of acetone, raise the temperature to 60°C and stir to continue the reaction until the NCO is lower than 0.1wt%, add 187g of acetone to dilute, and add 6.8g of triethylamine to neutralize for about 5min, then add 629g of water to the The mixture dispersed. After separation of acetone by distillation, a solvent-free aqueous dispersion of hydroxyl-containing polyurethane was obtained, which had a solids content of 35% by weight, an OH ...
Embodiment 2
[0093] Add 50g of dehydrated poly-1,6-hexanediol phthalate diol 1, 120g of dehydrated vegetable oil modified polyol, 88g of TDI, 28.05g of HMDI, 30g of WANOL R2307, 47g of acetone into a In a 1L four-neck round bottom flask with nitrogen inlet and outlet, the mixture was stirred at 80-90°C until NCO reached 4.78wt%. Cool down and add 10.0 g of dimethylolpropionic acid and 20 g of acetone, raise the temperature to 70° C. and stir to continue the reaction until the NCO reaches 2.82 wt%. Lower the temperature and add 33.5g trimethylolpropane and 106.4g acetone, heat up to 60°C and stir to continue the reaction until the NCO is lower than 0.1wt%, add 260g acetone to dilute, and add 6.8g triethylamine to neutralize for about 5min, then add 701g water to The mixture dispersed. After separation of acetone by distillation, a solvent-free aqueous dispersion of hydroxyl-containing polyurethane was obtained, which had a solids content of 35% by weight, an OH content of 2.5% by weight (b...
Embodiment 3
[0095] Add 120g of dehydrated vegetable oil-modified polyol 1, 90g of TDI, 19g of HMDI, 54g of WANOL R2307, and 42g of acetone into a 1L four-necked round-bottomed flask equipped with a nitrogen inlet and outlet, and stir the mixture at 80-90°C until the NCO reaches 4.06 wt%. Cool down and add 8g of dimethylolpropionic acid and 16g of acetone, raise the temperature to 70°C and stir to continue the reaction until the NCO reaches 2.35wt%. Cool down and add 26.2g of trimethylolpropane and 78.6g of acetone, heat up to 60°C and stir to continue the reaction until the NCO is lower than 0.1wt%, add 243g of acetone to dilute, and add 5.44g of triethylamine to neutralize for about 5min, then add 644g of water to The mixture dispersed. After separation of acetone by distillation, a solvent-free aqueous dispersion of hydroxyl-containing polyurethane was obtained with a solids content of 35% by weight, an OH content of 2.1% by weight (based on solids) in the dispersed phase by means of a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com