Clostridium septicum alpha toxin recombinant subunit vaccine and production method thereof
A subunit vaccine, Clostridium putrefaction technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, vaccines, etc., can solve the problems of local inflammation and toxicity, immune failure, immune effect decline, etc., to reduce biosafety risk effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
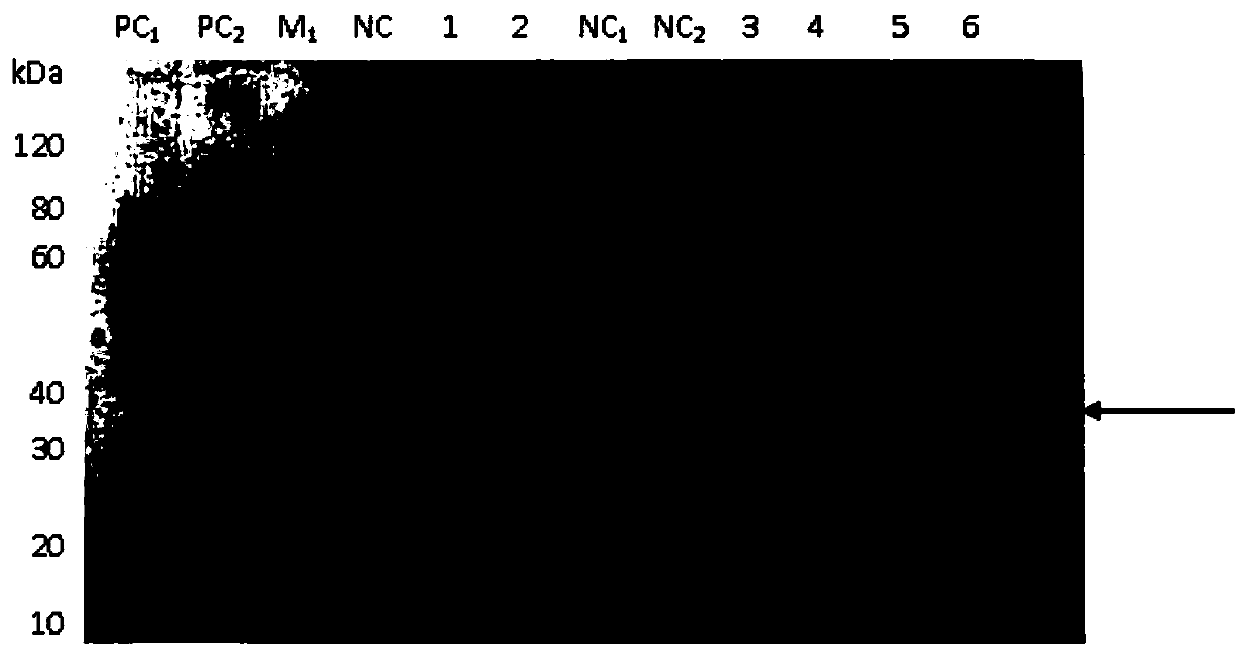
[0046] ——Construction, expression and identification of α-toxin 4 amino acid mutants of Clostridium putrefaciens
[0047] 1. Gene synthesis
[0048] According to the sequence of the natural protein gene of Clostridium putrefacilis α-toxin, this application designs 4 amino acid mutations after codon optimization, respectively mutating the 54th cysteine of the mature toxin of the wild-type Clostridium putrefactive α-toxin into leucine acid, asparagine at position 264 is mutated to alanine, histidine at position 269 is mutated to alanine, and tryptophan at position 310 is mutated to alanine, thus obtaining Clostridium putrefaciens which is non-toxic to animals Alpha toxin mutants. At the same time, a 6-histidine tag was added to the C-terminus of the mutant protein. The gene sequence was synthesized by chemical synthesis method, which contains 1254 nucleotides in total. Among them, positions 1-1233 are the mature toxin sequence of Clostridium putrefaction alpha toxin (includ...
Embodiment 2
[0064] ——Toxicity test of α-toxin 4 amino acid mutant of Clostridium putrefaciens to mice
[0065] The toxicity of the α-toxin 4 amino acid mutant of Clostridium putrefaction to mice was determined to verify the actual attenuation effect of the mutant in vivo. The Clostridium putrefaction α-toxin 4 amino acid mutant recombinant protein mCSA activated by Clostridium putrefaction α-toxin 4 amino acid mutant, and the wild-type Clostridium putrefaction α-toxin were inoculated into 16-18g mice via the tail vein at different doses, each Inject 5 rats at a dose of 0.2ml / rat. Results When the inoculation dose was 0.1 mg, all the mice were alive and without adverse reactions, while the wild-type control group could cause 5 / 5 mice to die when inoculated with 10 ng. This result indicated that the C. putrefaciens alpha toxin mutant was avirulent in mice and was identified as a toxin avirulent mutant.
[0066] Table 1 Toxicity of recombinant protein mCSA to mice
[0067]
Embodiment 3
[0069]——Immunogenicity test of α-toxin 4 amino acid mutants of Clostridium putrefaciens
[0070] (1) Bacterial liquid culture: the Escherichia coli BL21 / mCSA strain culture liquid of the recombinant expression Clostridium putrefaction α toxin mutant protein is inoculated with LB liquid culture containing kanamycin by 2% of the total amount of the culture medium base, cultured in a fermenter. The culture parameters were set as follows: culture temperature 37°C, pH value 7.0, dissolved oxygen 40%. when culture OD 600 When the value is 10-15, lower the temperature to 28°C, and add IPTG with a final concentration of 0.3mmol / L to induce culture for 4h.
[0071] (2) Bacteria destruction: collect the bacteria by centrifugation, add 10ml of lysate (pH 7.2 0.02mol / LTris buffer, 0.3mol / L NaCl) to resuspend the bacteria according to the wet weight of each gram of the bacteria, and resuspend the bacteria under the conditions of 4 Use a low-temperature high-pressure homogenizer to disru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com