A kind of preparation method of reactive gemini quaternary ammonium salt leather fungicide
A technology of gemini quaternary ammonium salt and bactericide is applied in the field of preparation of reactive gemini quaternary ammonium salt leather bactericide, can solve problems such as high toxicity, and achieve the effect of promoting bactericidal effect, significant bactericidal effect and reducing tanning cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
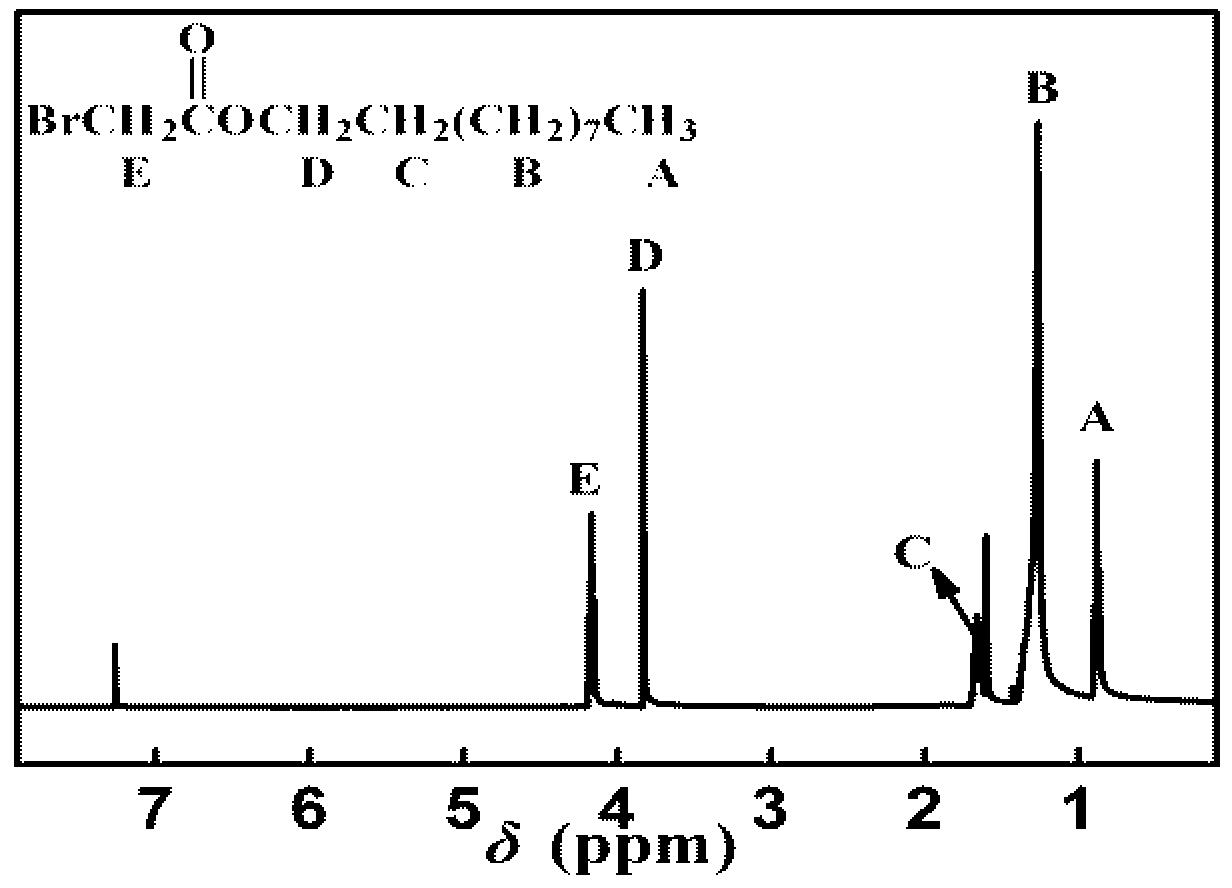
[0028] (1) Preparation of n-octyl bromoacetate:
[0029] To a 250 mL three-necked flask, add 4.84 g (24 mmol) of bromoacetyl bromide, 2.61 g (20 mmol) of n-octanol, 0.49 g (4 mmol) of catalyst and 30 mL of dichloromethane, react at room temperature for 2~3 h, add 20 mL The excess bromoacetyl bromide was removed with distilled water, the organic phase was extracted with dichloromethane, the crude product was obtained by rotary evaporation of the organic phase, and further purified by flash column chromatography (petroleum ether:ethyl acetate=20:1) to obtain n-octyl bromoacetate (4.37 g), 87% yield.
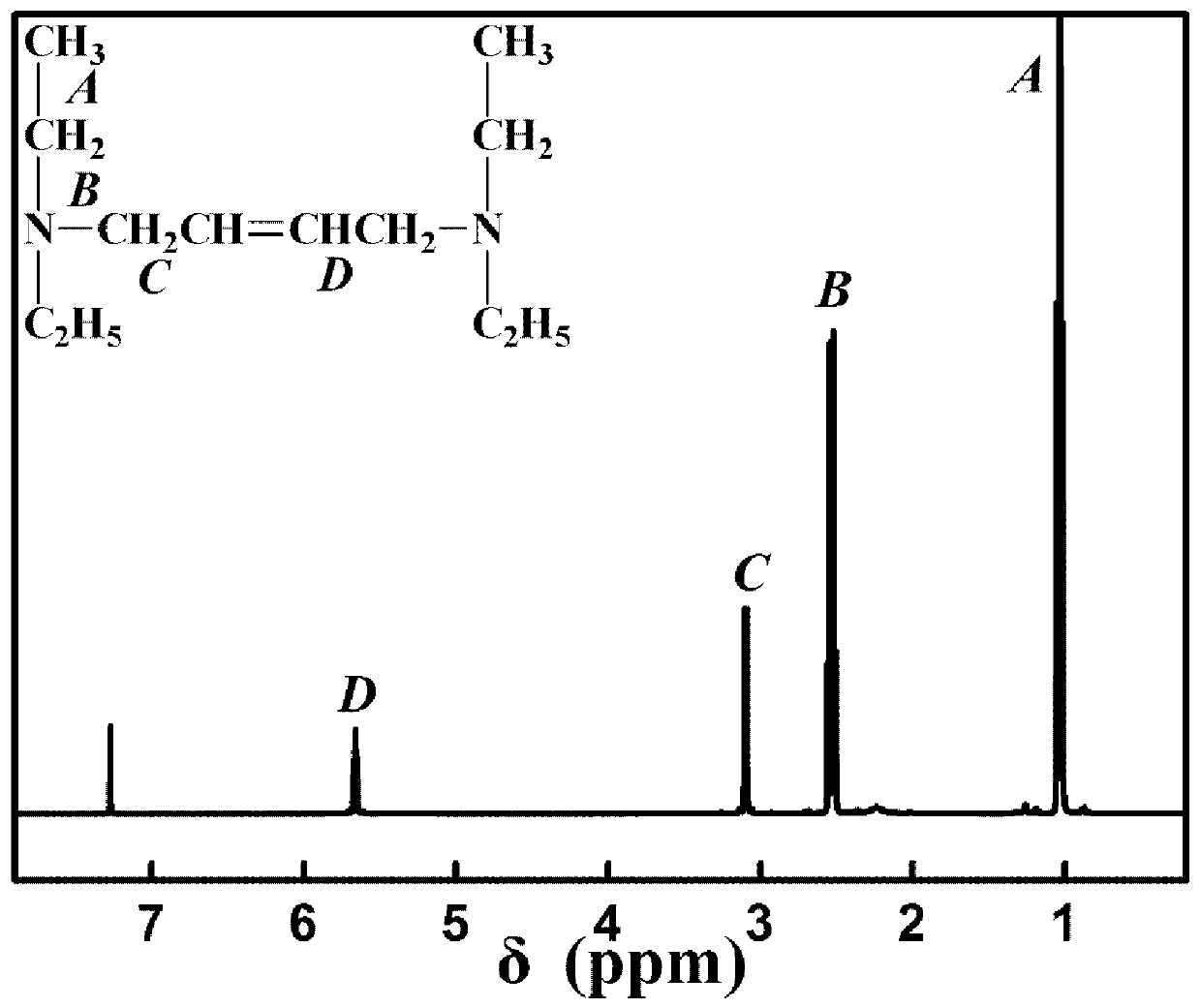
[0030] (2) Preparation of trans-N,N,N′,N′-tetraethyl-2-butene-1,4-diamine
[0031]To a 250 mL three-necked flask, 0.19 g (15 mmol) of trans-1,4-dichloro-2-butene, 16.26 g (225 mmol) of diethylamine and 30 mL of solvent were added, and the reaction was carried out at 50 to 70 °C for 3 to 5 h. The precipitate was removed by filtration, and the crude product was obtained by rotary e...
Embodiment 2
[0035] (1) Preparation of n-decyl bromoacetate:
[0036] To a 250 mL three-necked flask, add 4.84 g (24 mmol) of bromoacetyl bromide, 3.17 g (20 mmol) of n-decanol, 0.49 g (4 mmol) of catalyst and 30 mL of dichloromethane, react at room temperature for 2~3 h, add 20 mL The excess bromoacetyl bromide was removed with distilled water, the organic phase was extracted with dichloromethane, the crude product was obtained by rotary evaporation of the organic phase, and further purified by flash column chromatography (petroleum ether:ethyl acetate=20:1) to obtain n-decyl bromoacetate (4.96 g), 88.8% yield.
[0037] (2) Preparation of trans-N,N,N′,N′-tetraethyl-2-butene-1,4-diamine
[0038] To a 250 mL three-necked flask, add 0.19 g (15 mmol) of trans-1,4-dichloro-2-butene, 16.26 g (225 mmol) of diethylamine and 30 mL of solvent, and react at 50~70°C for 3~5 h , filtered to remove the precipitate, and the crude product was obtained by rotary evaporation of the filtrate, which was fu...
Embodiment 3
[0042] (1) Preparation of n-dodecyl bromoacetate:
[0043] To a 250 mL there-necked flask, add 4.84 g (24 mmol) of bromoacetyl bromide, 3.73 g (20 mmol) of n-dodecanol, 0.49 g (4 mmol) of catalyst and 30 mL of dichloromethane solvent, and react at room temperature for 2~3 h, 20 mL of distilled water was added to remove excess bromoacetyl bromide, the organic phase was extracted with dichloromethane, the crude product was obtained by rotary evaporation of the organic phase, and further purified by flash column chromatography (petroleum ether:ethyl acetate=20:1) to obtain bromoacetic acid n-dodecyl ester (5.47 g), 89% yield.
[0044] (2) Preparation of trans-N,N,N′,N′-tetraethyl-2-butene-1,4-diamine
[0045] To a 250 mL three-necked flask, 0.19 g (15 mmol) of trans-1,4-dichloro-2-butene, 16.26 g (225 mmol) of diethylamine and 30 mL of solvent were added, and the reaction was carried out at 50 to 70 °C for 3 to 5 h. The precipitate was removed by filtration, and the crude produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com