Inactivated lactic acid bacteria vaccine adjuvant
A technology for inactivating lactic acid bacteria and vaccine adjuvants, which is applied in the field of inactivated lactic acid bacteria vaccine adjuvants, and can solve the problems of high safety, efficacy to be improved, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Inoculate Enterococcus faecium (purchased from China Industrial Microorganism Culture Collection and Management Center, Latin name: Enterococcusfaecium, preservation number: CICC 6049) in MRS medium, cultivate in an incubator at 37°C for 24 hours, then centrifuge at 3000 rpm for 5 minutes, remove Keep the precipitate in the upper layer culture solution, add sterile normal saline to wash the precipitate, centrifuge for 5 minutes, repeat the washing 3 times, add sterile normal saline, and mix with the precipitate. Get a certain amount of Enterococcus faecium physiological salt suspension solution, measure its OD value at the 690nm place of the spectrophotometer, when the OD value of the final concentration diluted with sterile physiological saline is 0.38, the Enterococcus faecium of such diluted concentration Physiological saline solution is used as 1 times (1×) concentration, and it can be determined that each ml of bacterial suspension at this concentration contains ~10...
Embodiment 2
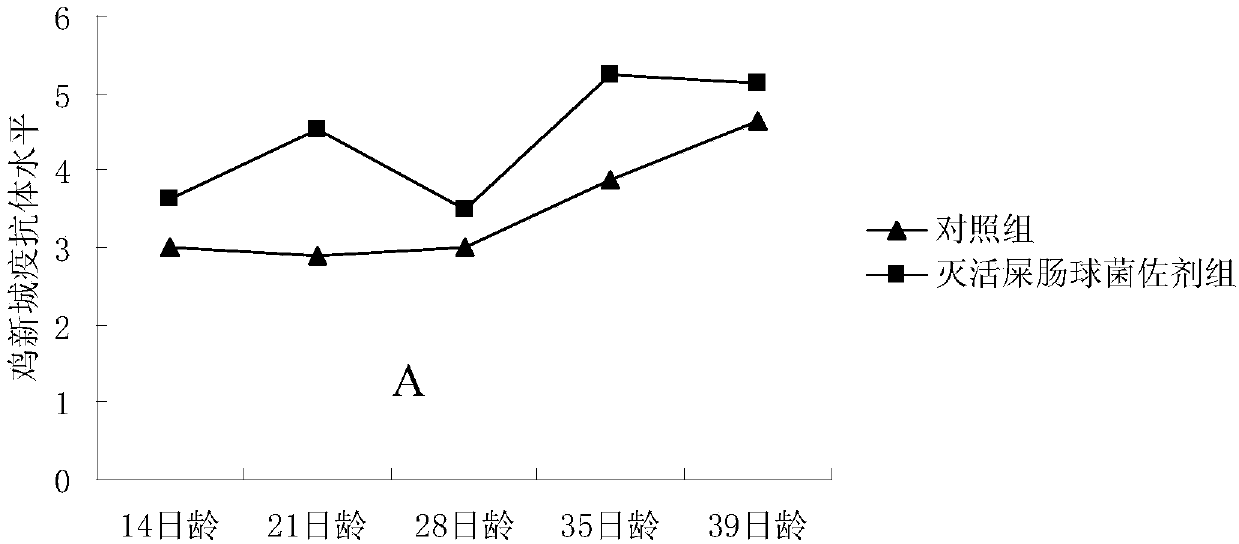
[0024] Dilute the above-prepared inactivated Enterococcus faecium suspension to a concentration of 10×, and detect the effect of inactivated Enterococcus faecium as an adjuvant on the antibody levels of Newcastle disease and avian influenza H9 subtypes after chickens were inoculated with Xinliu dual inactivated vaccine . Twenty white-feathered broilers were randomly divided into two groups, namely the control group and the inactivated Enterococcus faecium adjuvant group, with 10 chickens in each group. Vaccine immunization was carried out at the age of 7 days. When the control group was vaccinated, 20 mL of sterile saline was added to 30 mL of Xinliu double inactivated oil seedlings and mixed well, and 0.5 mL was injected subcutaneously into the neck of each chick; When the coccal adjuvant group was vaccinated, 20 mL of inactivated Enterococcus faecium suspension at a concentration of 10× was added to 30 mL of Xinliu double inactivated oil seedlings, mixed evenly, and 0.5 mL w...
Embodiment 3
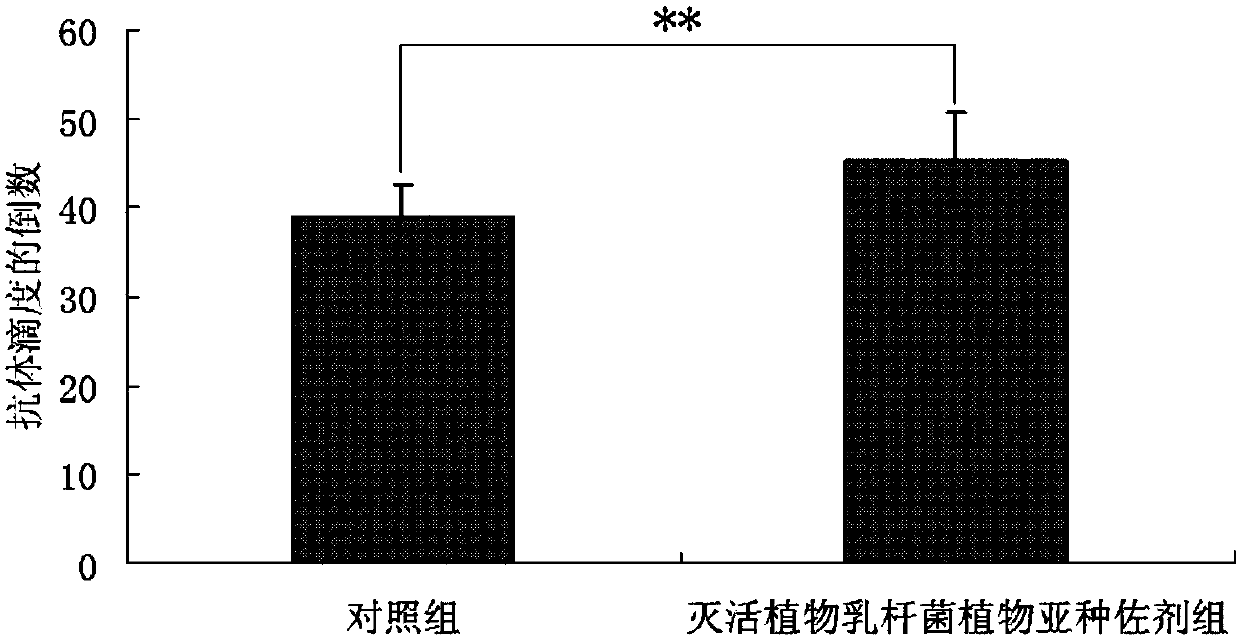
[0026] Lactobacillus plantarum subsp. plantarum (purchased from China Industrial Microorganism Culture Collection and Management Center, Latin name: Lactobacillus plantarum subsp.Plantarum, preservation number: CICC 6240) was prepared according to the method described in Example 1 into a 10× concentration of inactivated plant Lactobacillus subsp. plantarum vaccine adjuvant. To detect the effect of inactivated Lactobacillus plantarum subsp. plantarum as an adjuvant on the antibody level of mice inoculated with classical swine fever vaccine. Twenty clean-grade Kunming mice weighing 18-22 g were randomly divided into two groups, namely the control group and the inactivated Lactobacillus plantarum subsp. plantar adjuvant group, with 10 mice in each group, half male and half male. When the mice in the control group were inoculated with the live CSF vaccine, an equal volume of sterile saline was added to the live CSF vaccine; 100 μL was injected intramuscularly on the outer side of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com