2,3-epoxy-2-propylsulfone-5,8-dimethoxy-1,4-naphthoquinone, its preparation method and medicine containing it
A dimethoxy, naphthoquinone technology, applied in the field of medicine, can solve the problems of lack of discrimination ability of chemotherapy, decreased blood picture, liver function damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
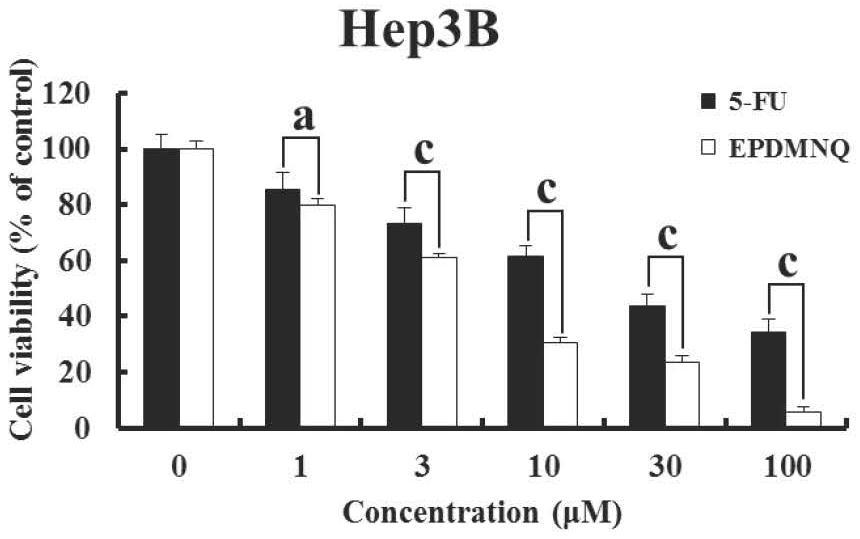
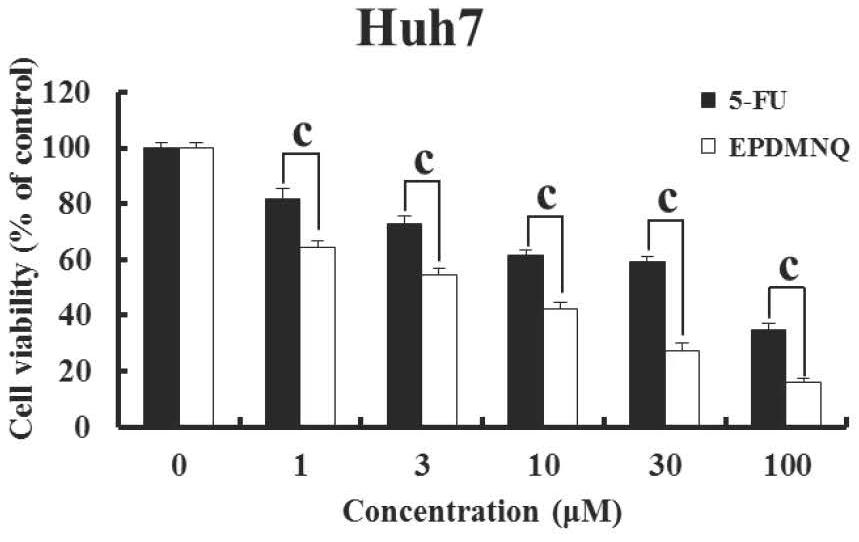
Image
Examples
Embodiment
[0054] Example: Preparation of 2,3-epoxy-2-propylsulfone-5,8-dimethoxy-1,4-naphthoquinone compound in this example:
[0055] 1. Synthesis of 2-propylmercapto-5,8-dimethoxy-1,4-naphthoquinone
[0056] In a 100ml reaction bottle, add 218.21mg (1mmol) of 5,8-dimethoxy-1,4-naphthoquinone and 50ml of methanol, mix well, add 111.02μl (1.5mmol) of 1-propanethiol, and react at room temperature for 4 hours Finally, 149.8 mg (0.2 mmol) of sodium dichromate and 27.2 μl (0.75 mmol) of concentrated sulfuric acid were added to the mixture, and the reaction was completed after 5-10 minutes. Extracted with dichloromethane and saturated brine, dried with an appropriate amount of anhydrous sodium sulfate, filtered, and concentrated to dryness to obtain a crude product, which was prepared by column chromatography, 2-propylmercapto-5,8-dimethoxy-1,4- naphthoquinone.
[0057] 2. Synthesis of 2,3-epoxy-2-propylsulfone-5,8-dimethoxy-1,4-naphthoquinone (EPDMNQ)
[0058] In a 50ml reaction bottle, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com