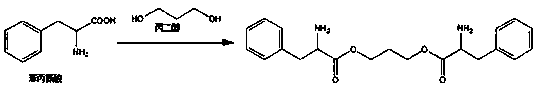
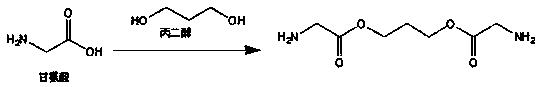
Degradation time controllable and breaking elongation adjustable medical degradable polyurethane
A technology of elongation at break and degradation time, applied in the field of medical degradable polyurethane with controllable degradation time and adjustable elongation at break, can solve problems such as slow degradation of polyurethane
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Weigh 9.0g ε-caprolactone, 3.0g PEG-600, and stannous octoate (0.03wt% of the total) as catalysts, add them to a vacuum reaction bottle, add a magnetic stirrer, and vacuumize / fill with nitrogen Circulate 3 times, and seal the vacuum reaction bottle mouth under vacuum conditions, put it in an oil bath at 140°C for 24 h to obtain a linear polymer. Then weigh 2 g of L-lysine diisocyanate and 0.6 g of glycine diamine, vacuumize and seal the bottle mouth, put them into an oil bath at 50° C. for 4 h to obtain the final product.
Embodiment 2
[0082] Weigh 9.0g ε-caprolactone, 0.5g PEG-600, and stannous octoate (0.03wt% of the total) as catalysts, add them to a vacuum reaction bottle, add a magnetic stirrer, and vacuumize / fill with nitrogen Circulate 3 times, and seal the vacuum reaction bottle mouth under vacuum conditions, put it into an oil bath at 140° C. for 18 hours to obtain a linear polymer. Then weigh 2.6g of L-lysine diisocyanate and 0.68g of phenylalanine diamine, vacuumize and seal the bottle mouth, and put them in an oil bath at 70°C for 8 hours to obtain the final product.
Embodiment 3
[0084] Weigh 9.0g ε-lactide, 1.0g PEG-200, and bismuth neodecanoate (1.0wt% of the total) as catalysts, add them into a vacuum reaction bottle, and then add a magnetic stirrer, vacuumize / fill Nitrogen was circulated 3 times, and the vacuum reaction bottle was sealed under vacuum conditions, and placed in an oil bath at 130°C for 24 h to obtain a linear polymer. Then weigh 2 g of L-lysine diisocyanate and 0.7 g of cysteine diamine, vacuumize and seal the bottle mouth, put them in an oil bath at 70° C. for 4 h to obtain the final product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com