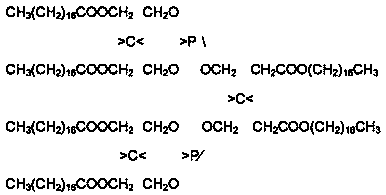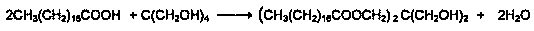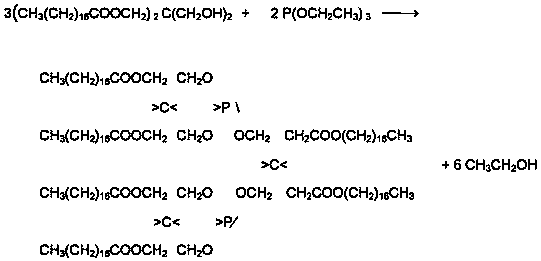Phosphorous acid containing organic ester anti-oxidant and synthetic method
A synthesis method, alcohol diphosphite technology, applied in the directions of organic chemistry, chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, etc. problems, to achieve the effect of improving color, high thermal stability, and good processing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] In a reaction flask equipped with electric stirring, electric heating, reflux condenser, and temperature measuring system, 67 grams of pentaerythritol, 279 grams of stearic acid, and 1 gram of catalyst stannous chloride were added to the reaction flask respectively, and protected under vacuum conditions. The esterification reaction was carried out at 150-220°C, and the reaction time was 150 minutes. After the reaction was completed, it was cooled to below 100°C, 54 grams of triethyl phosphite was added, and 2 grams of catalyst phosphoric acid was added, and the second reaction was carried out under the protection of nitrogen. Step-by-step reaction, control the reaction temperature at 140-200°C, and the reaction time is 150 minutes. After the reaction is completed, cool to below 100°C. The product is filtered through a constant temperature oven at about 80°C to obtain the product of organic phosphite antioxidant—tri-distearin Acid pentaerythritol diphosphite.
example 2
[0029] In a reaction flask equipped with electric stirring, electric heating, reflux condenser, and temperature measuring system, 50 grams of pentaerythritol, 209 grams of stearic acid, and 1.3 grams of catalyst phosphoric acid were added to the reaction flask respectively, and esterification was carried out under nitrogen protection. Reaction, control the reaction temperature at 130-220°C, and the reaction time is 180 minutes. After the reaction is completed, cool to below 100°C, then add 41 grams of triethyl phosphite, add 0.5 grams of catalyst phosphoric acid, and carry out the second step reaction under the protection of nitrogen. Control the reaction temperature at 140-200°C, and the reaction time is 150 minutes. After the reaction is completed, cool to below 100°C. The product is then filtered in a constant temperature oven at about 80°C to obtain the organic phosphite antioxidant product—pentaerythritol di-tri-distearate Phosphite.
example 3
[0031] In a reaction flask equipped with electric stirring, electric heating, reflux condenser, and temperature measuring system, 136 grams of pentaerythritol and 270 grams of palmitic acid were added to the reaction flask, and 3.4 grams of catalyst isopropyl titanate were carried out under nitrogen protection. For esterification reaction, control the reaction temperature at 170-220°C, and the reaction time is 180 minutes. After the reaction is completed, cool to below 100°C, add 58 grams of triethyl phosphite, and add 1 gram of catalyst isopropyl titanate, under the protection of nitrogen Carry out the second step of reaction, control the reaction temperature at 150-210°C, and the reaction time is 180 minutes. After the reaction is completed, cool down to below 100°C. The product is filtered through a constant temperature oven at about 80°C to obtain the product of organic phosphite antioxidant—three- Pentaerythritol Diphosphite Dipalmitate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com