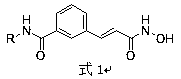
Belinostat structural analogue with histone deacetylase inhibiting effect and application of belinostat structural analogue
A sirupin and belistat technology, applied in the field of belistat's structural analogues, can solve the problem of unrecorded sulfonamide bond replacement HDACIs etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
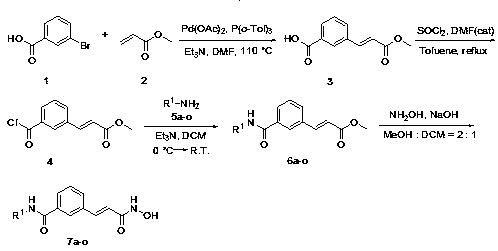
[0024] Synthesis of 3-(2-(methoxyformyl)vinyl)benzoic acid (3)
[0025] Dissolve m-bromobenzoic acid (3.0g, 15mmol) and methyl acrylate (6.45g, 75mmol) in N,N-dimethylformamide (50mL), add triethylamine (202mg, 2mmol), palladium acetate ( 67mg, 0.3mmol) and three (o-methylphenyl) phosphorus (92mg, 0.3mmol). Under nitrogen, the temperature of the reaction solution was raised to 110° C. for 5 hours. Then cool to room temperature, adjust the pH value to about 2 with dilute hydrochloric acid, filter the precipitated solid, and recrystallize with acetone to obtain 2.15 g of white solid, with a yield of 70%. 1 H NMR (400MHz, DMSO-d 6 )δ8.20(d, J=1.8Hz, 1H), 8.02-7.95(m, 2H), 7.74(d, J=16.1Hz, 1H), 7.56(t, J=7.7Hz, 1H), 6.72( d,J=16.1Hz,1H),3.74(s,3H).
Embodiment 2
[0027] Synthesis of 3-(2-(methoxyformyl)vinyl)benzoyl chloride (4)
[0028] 3-(2-(Methoxyformyl)vinyl)benzoic acid 3 (1.03g, 5mmol) was dissolved in an appropriate amount of toluene, and thionyl chloride (1.1mL, 15mmol) and 1 drop of N,N-dimethyl Formamide. After reacting at 90°C for 1 hour, toluene and excess thionyl chloride were distilled off under reduced pressure to obtain acid chloride 4. The crude product was used directly in the next step without purification.
Embodiment 3
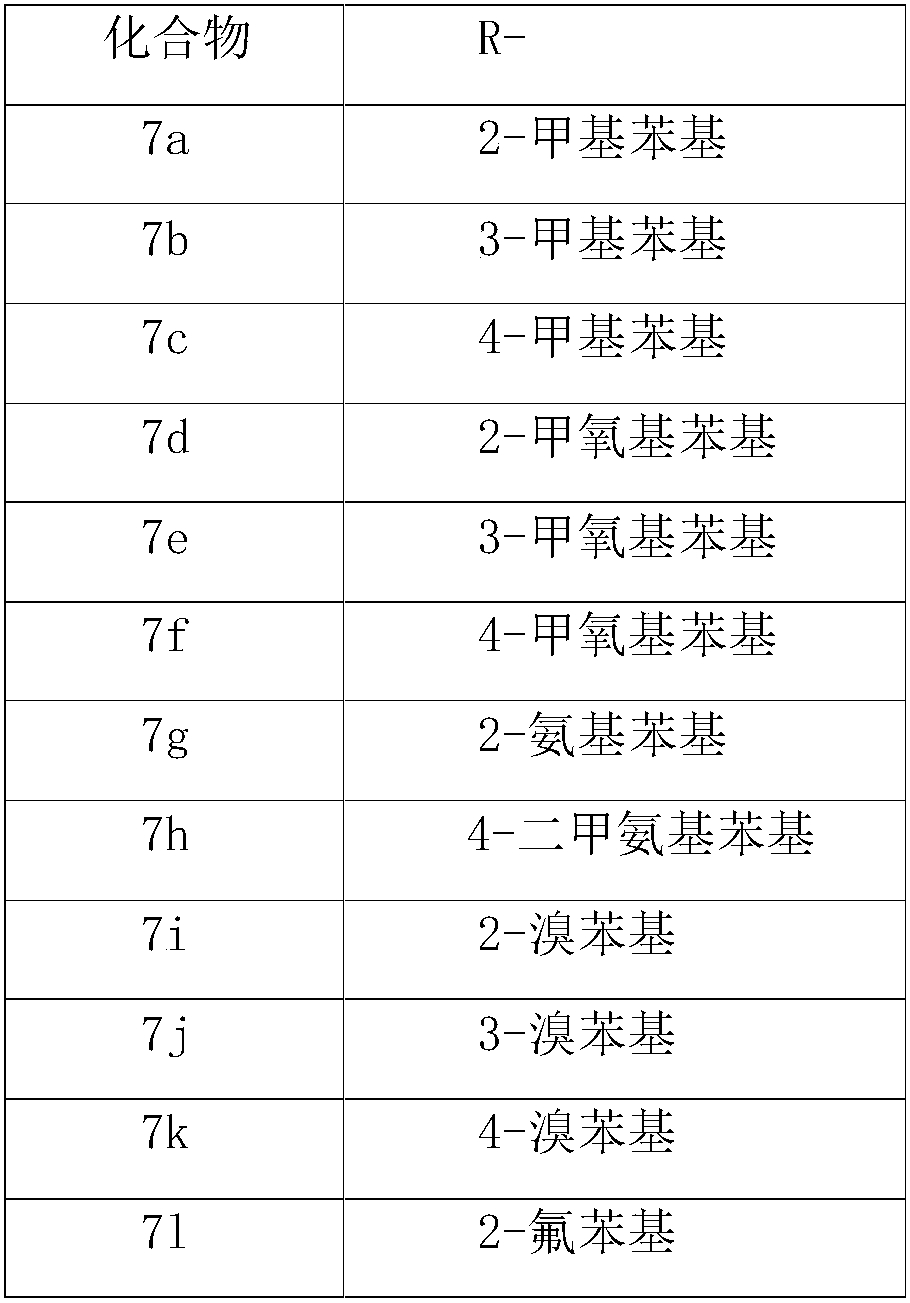
[0030] General Synthesis of Methyl 3-(3-(Substituted Aryl)carbamoyl)Benzacrylate (6)
[0031] Substituted arylamines 5a-o (5mmol) were dissolved in dichloromethane (20mL), cooled to 0°C in an ice-water bath, triethylamine (7mmol) was added, and then a solution of compound 4 in dichloromethane was slowly added dropwise. The reaction solution was slowly raised to room temperature and stirred for 1-2 hours until compound 4 disappeared (monitored by TLC). After the reaction, the pH value was adjusted to about 2 with dilute hydrochloric acid. The organic layer was separated, the aqueous layer was extracted with dichloromethane, the organic layers were combined, washed with water, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. Separation by column chromatography (petroleum ether: ethyl acetate = 3:1) gave compounds 6a-o.
[0032] Methyl 3-(3-(2-methylphenyl)carbamoyl)benzoacrylate (6a)
[0033] White solid, yield 35%. 1 H NMR (400M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com