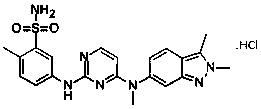
Pazopanib hydrochloride as well as preparation method and application thereof
A technology of pazopanib hydrochloride and pazopanib hydrochloride crystals, applied in the field of crystals of pazopanib hydrochloride and its preparation, can solve the problems of high impurity content, poor method reproducibility, content and crystal form cannot be reproduced, etc. problems, to achieve high-purity, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
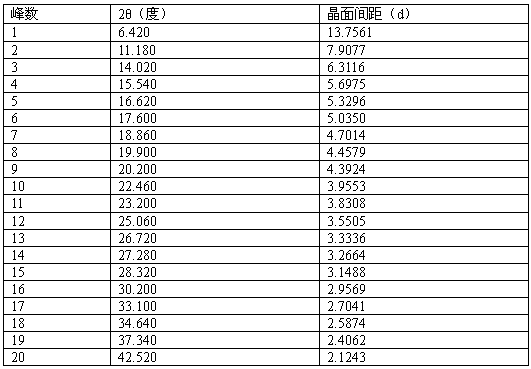
[0031] In a 50L reactor, add 3kg of pazopanib hydrochloride (purity 97.3%, HPLC) and 30.3L of water-acetone-ammonia=9:0.8:1.1 mixture, heat to 60°C-72°C, add 150 grams of activated carbon were incubated and stirred for 30 minutes, filtered while hot, naturally cooled to room temperature under filtrate stirring, and then incubated for 5.5 hours, crystallized, filtered, and vacuum-dried at room temperature to obtain 2.81 kilograms of pazopanib hydrochloride crystals. Melting point: 290.5°C-300.6°C, purity 99.95%, single impurity 0.04%, MS: 349.13 (M+H) solvent residue detection meets the requirements.
Embodiment 2
[0033] Capsules Containing Pazopanib Hydrochloride Compound
[0034] Prescription: 85 grams of pazopanib hydrochloride, 3.5 ml of propylene glycol, 145 grams of starch, made into 1000 capsules.
[0035] Process: Wet pazopanib hydrochloride and starch with 20% propylene glycol aqueous solution, mix well, sieve and granulate, dry at 55°C, granulate, and fill capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com