Preparation method of muscone
A technology of musk ketone and ketal is applied in the field of preparation of musk ketone, which can solve the problems of complicated preparation process, high cost, limitation of industrial mass production of musk ketone, etc., and achieve the effects of simple process and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] see figure 1 , a preparation method of muscone, wherein, comprises the steps:
[0028] (1) Preparation of the first ketal compound attached to the polystyrene resin:
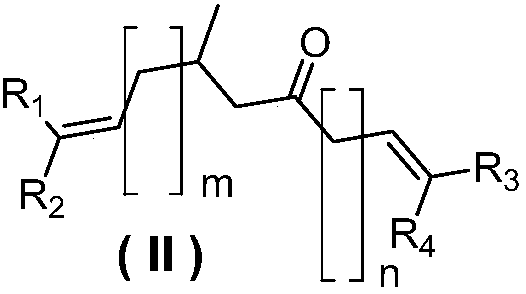
[0029] In a 50L glass reactor equipped with a thermometer, a mechanical stirrer, and a water separator, add 2kg of polystyrene resin-loaded 1,2-diol (I), the second preset mass (1.28kg or 1.38kg) of 3 -Methyldiketene (II), 20L of toluene and the first catalyst of the third preset mass (4.4g or 7.3g), stirring and heating to 40°C-140°C, and reacting for the first preset time (2~20h ) and cooled to room temperature, filtered to obtain the first ketal compound loaded on polystyrene resin, wherein the first ketal compound is 2,6-dimethyl-octadeca-2,17-diene-8- Ketal or 9-methylheptadeca-1,16-diene-7-ketal.
[0030] Among them, the structural characteristics of the 1,2-diol (k is an integer from 4 to 18) carried by the polystyrene resin are:
[0031]
[0032] The chain length of the 1,2-diol and polysty...
Embodiment 2
[0047] (1) Preparation of 2,6-dimethyl-octadeca-2,17-diene-8-ketal supported on polystyrene resin (shown below, k=9):
[0048]
[0049] In a 50L glass reactor equipped with a thermometer, a mechanical stirrer and a water separator, add 2kg of 1,2-diol and 1.28kg of 2,6-diol with a degree of substitution of 0.73mol / kg polystyrene resin Methyl-octadec-2,17-dien-8-one, 20 L of toluene and 7.3 g of pyridinium p-toluenesulfonate. Heating to reflux and separating water for 10h under stirring conditions. After being cooled to room temperature, filter, and the filtrate obtained by filtering is used mechanically in the next reaction, and the polystyrene resin is washed 3 times with dichloromethane (10 liters each time), and is used in the next step.
[0050] (2) Preparation of 3-methylcyclopenta-6-ene ketal attached to polystyrene resin:
[0051]
[0052] In a 50L glass reactor equipped with a thermometer, mechanical stirring and a reflux condenser, add the 2,6-dimethyl-octadec...
Embodiment 3
[0060] (1) Resin preparation of 9-methylheptadeca-1,16-diene-7-ketal attached to polystyrene resin (as shown below, k=13):
[0061]
[0062] In a 50L glass reactor equipped with a thermometer, a mechanical stirrer and a water separator, add 2kg of 1,2-diol carried by polystyrene resin with a degree of substitution of 0.87mol / kg, 1.38kg of 9-methyl Heptadeca-1, 16-dien-7-one, 20 L of toluene and 4.4 g of pyridinium p-toluenesulfonate were heated under reflux to separate water for 15 hours while stirring. Filter after cooling to room temperature, and use the filtrate obtained by the filtration for the next reaction. The polystyrene resin is washed 3 times with dichloromethane (10 liters each time), and it will be used in the next step.
[0063] (2) Preparation of 3-methylcyclopenta-9-ene ketal attached to polystyrene resin:
[0064]
[0065] In a 50L glass reactor equipped with a thermometer, mechanical stirring and a reflux condenser, add the 9-methylheptadeca-1,16-diene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com