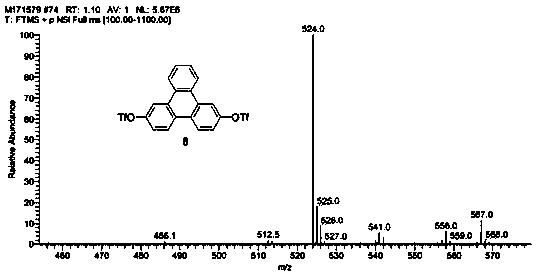
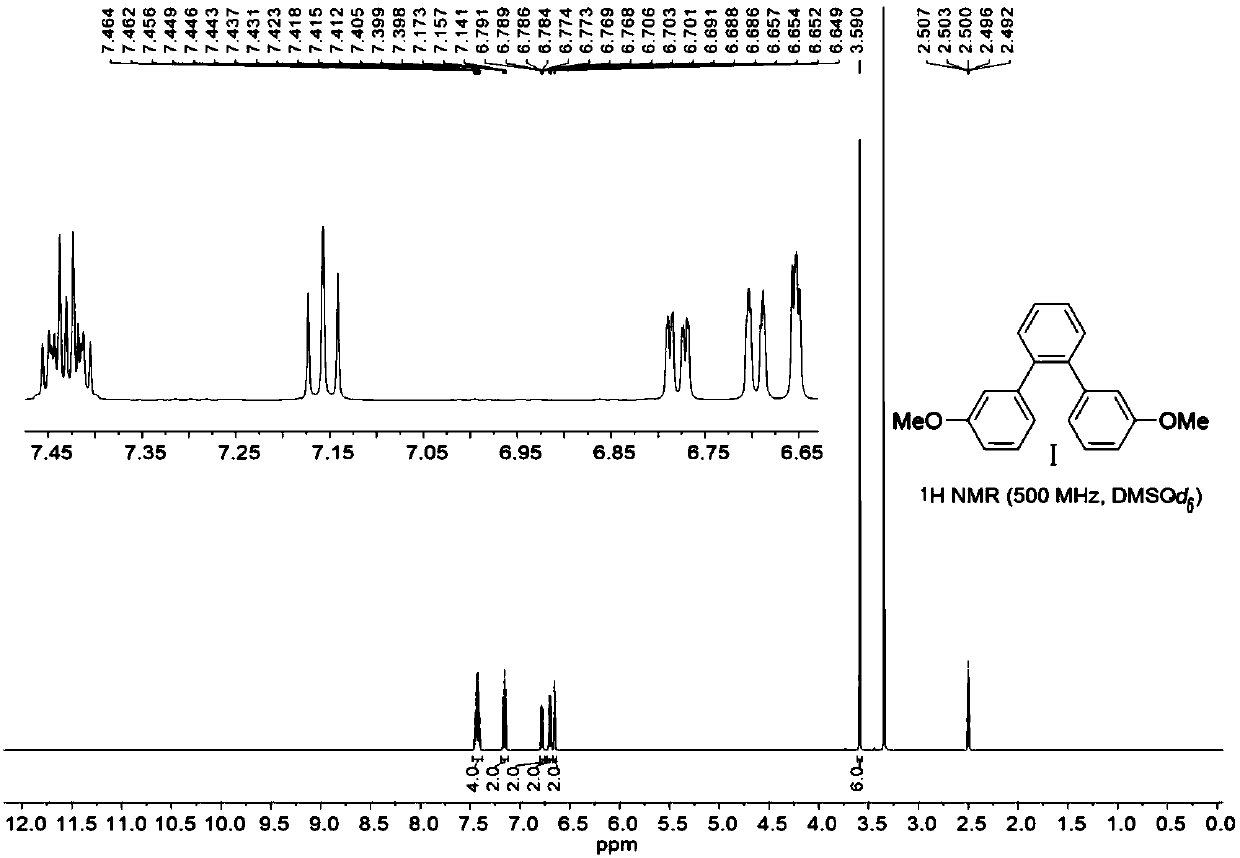
Preparation method of key intermediate of triphenylene bisborate
A technology for triphenylene bis-boronate and intermediates, which is applied to the preparation of sulfonate esters, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of low preparation cost, low yield of oxidative coupling, and the problem of starting raw materials Expensive and other issues, to achieve the effect of increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0067] The present invention will be further described below through specific examples, but the protection scope of the present invention is not limited thereto.
[0068] Step 1: Preparation of 1,2-bis(3-methoxyphenyl)benzene (Compound 1)
[0069]
[0070] Add 3-methoxyphenylboronic acid (28.80 g, 189 mmol, 3.00 equivalents), Pd (PPh 3 ) 4 (2.91g, 2.52mmol, 0.05eq) and Na 2 CO 3 (40.10 g, 378.00 mmol, 6.00 equiv). Nitrogen was purged three times, then 1,2-dibromobenzene (7.50 mL, 63.00 mmol, 1.00 equiv), toluene (150 mL), deionized water (150 mL) and ethanol (150 mL) were added. Then nitrogen bubbles were bubbled through for 30 minutes, and the reaction mixture was refluxed for 2 days. Then cool to room temperature, filter, wash the filter handle (100mL×3) with ethyl acetate, concentrate, dilute with a large amount of ethyl acetate, then wash with 3M hydrochloric acid, separate the organic phase, and wash with NaHCO 3 Wash until no bubbles are generated, separate the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com