Nickel-based catalyst for reversed water gas shift reaction and preparation method of nickel-based catalyst
A nickel-based catalyst, a technology for shift reaction, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Catalytic activity, reducing the loss of specific surface area, preventing the effect of ionic surface diffusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
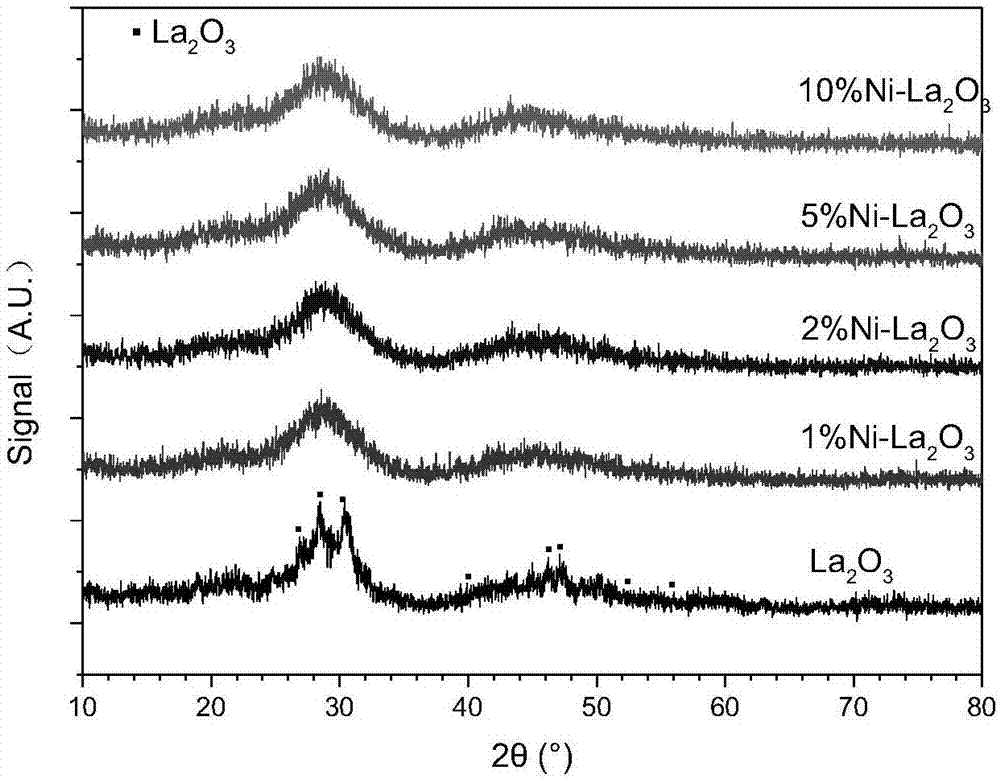
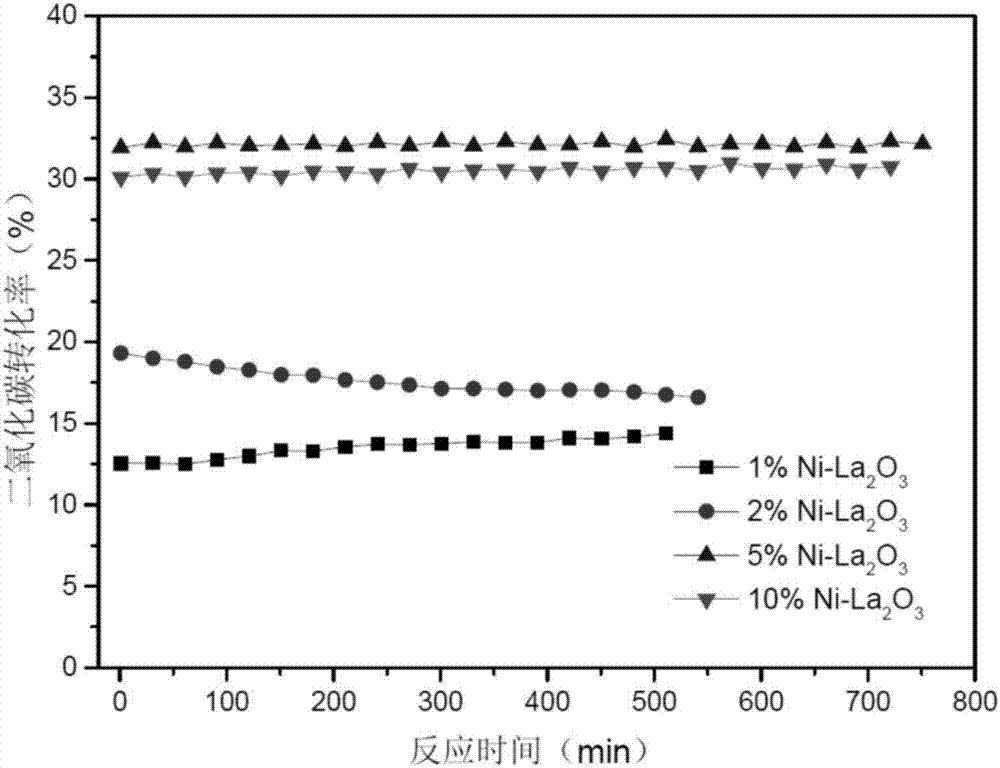
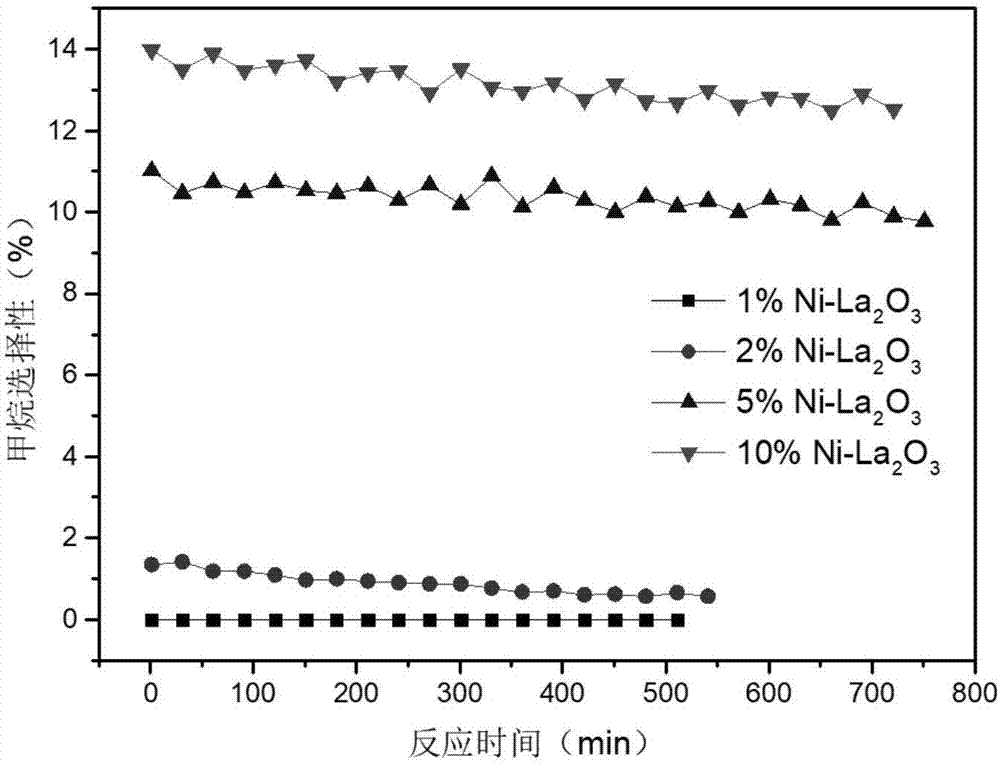
Image
Examples
Embodiment 1
[0026] Weigh 0.0496g of Ni(NO 3 ) 3 ·6H 2 O, 2.6314 La(NO 3 ) 3 ·6H 2 O, 0.6g glycine, measure 6.3ml of H 2 O, sonicate for 20 minutes until completely dissolved. Add 1.0ml of silica sol (neutral), and ultrasonically vibrate for 20min. Pour the above solution into a 250ml beaker, and heat it to 230°C on a hot plate until the glycine and nitrate react completely. After cooling to room temperature, in the muffle furnace, the heating rate was set at 2°C / min, and the temperature was increased from room temperature to 600°C for calcination for 4h. The obtained calcined product was dissolved in 2 mol / L NaOH solution, and stirred at 80° C. for 4 h under constant magnetic force. Multiple centrifugal washings (water→water→ethanol→water→ethanol) yielded solid powder. Transfer to a small beaker, dry in a blast oven at 100°C for 6 hours, and cool to room temperature to obtain 1% Ni-La 2 o 3 .
Embodiment 2
[0028] In addition to Ni(NO 3 ) 3 ·6H 2 The amount of O is 0.1g, La(NO 3 ) 3 ·6H 2 The amount of O is 2.60g, and the rest of the preparation method is exactly the same as in Example 1 to obtain 2%Ni-La 2 o 3 .
Embodiment 3
[0030] In addition to Ni(NO 3 ) 3 ·6H 2 The amount of O is 0.248g, La(NO 3 ) 3 ·6H 2 The amount of O is 2.53g, and the rest of the preparation method is exactly the same as in Example 1 to obtain 5% Ni-La 2 o 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com