Injectable high-strength chitin-based hydrogel, and preparation method and application thereof
A hydrogel and chitin technology, applied in the field of biomedical materials and tissue engineering, to achieve wide application prospects, good biocompatibility, easy to obtain or prepare effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] Preparation of Hydrophilic Polymer A
[0043] (1) Synthesis of linear diester alkyne-terminated PEG (DA-PEG)
[0044]
[0045] Weigh 10.0 grams of PEG (M n =2000) and 1.05 gram of propiolic acid were placed in a 250mL round-bottomed flask, 150mL of dried toluene solution was added as a solvent, and 0.57 gram of p-toluenesulfonic acid was added as a catalyst under magnetic stirring, and the oil bath was heated to 140°C , condensed and refluxed, the whole system reacted for 48h and then cooled to room temperature. The product mixed solution was concentrated and precipitated in ether, and the collected crude product was recrystallized and dried to obtain 9.33 g of light yellow solid as linear diester alkyne terminal PEG (DA-PEG), with a yield of 88.6%. 1 H NMR (500MHz, CDCl 3 , δ): 4.3(m, COOC H 2 ), 3.7 (m, COOCH 2 C H 2 O), 3.6(m, PEG main chain OC H 2 C H 2 O), 3.0 (s, C H ≡CCOO). By choosing linear PEGs with different molecular weights as raw materials...
Embodiment 1
[0055] Preparation of DA-PEG / CM-Chitosan hydrogel by spontaneous amine-alkyne click reaction.
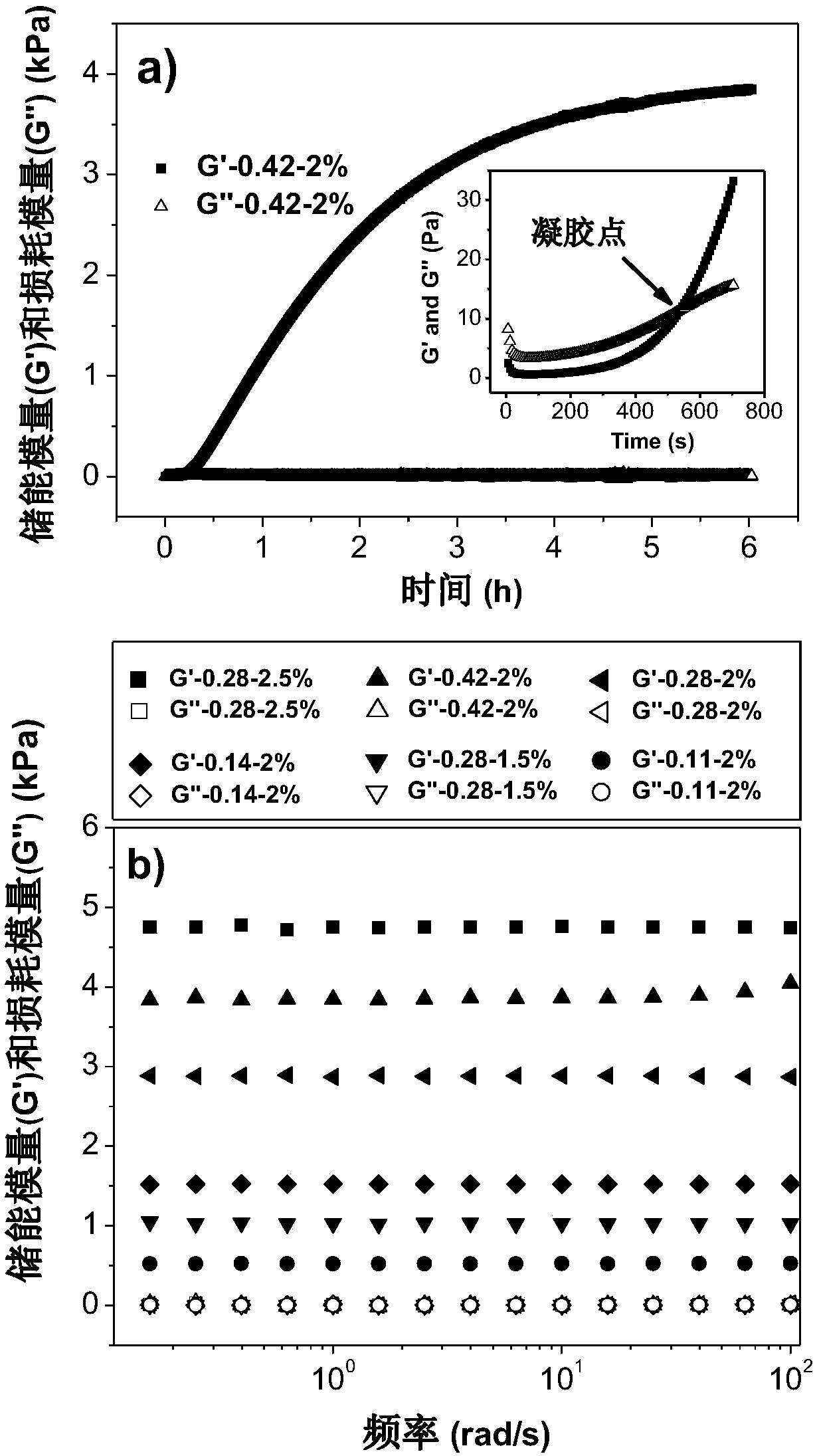
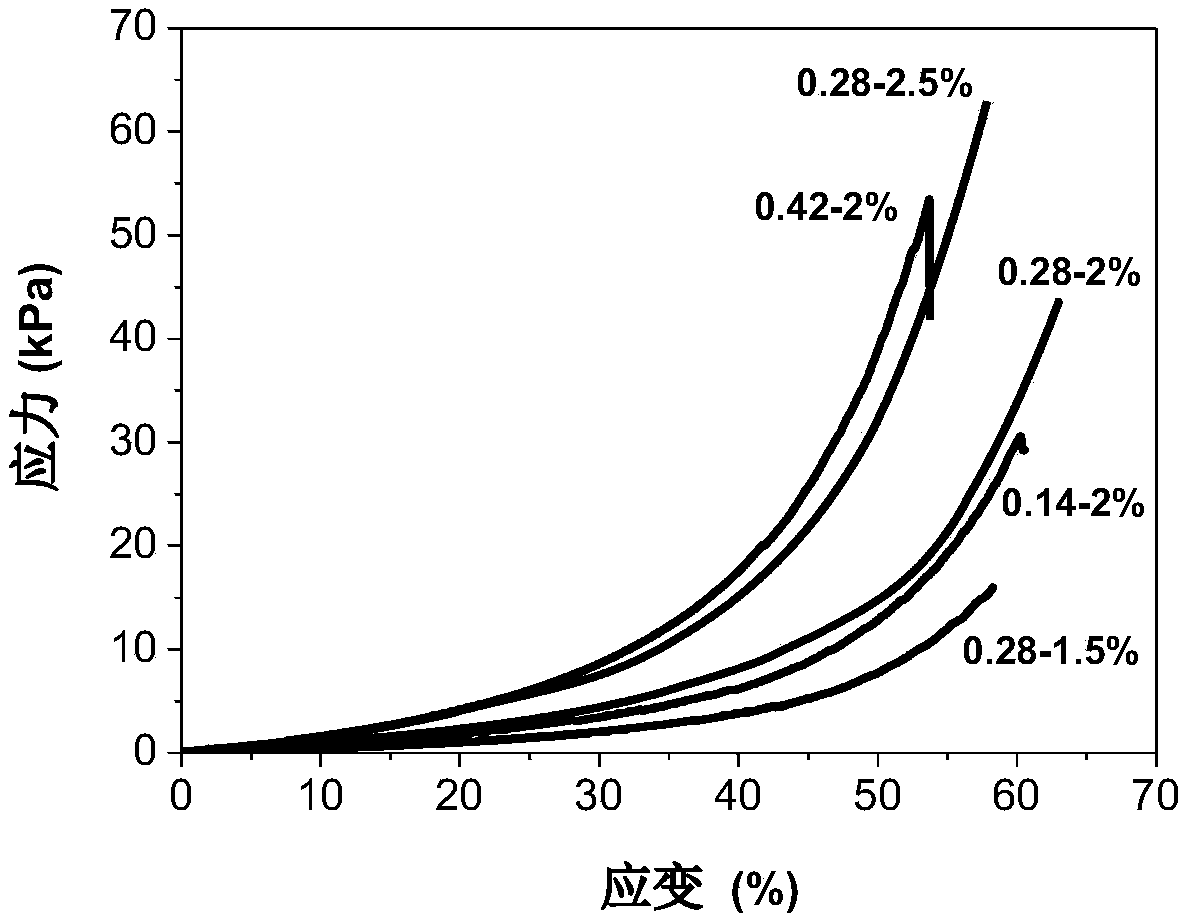
[0056] DA-PEG (PEG molecular weight 2000) and carboxymethyl chitosan (CM-Chitosan, weight average molecular weight 74kDa, deacetylation degree 0.96, carboxymethyl substitution degree 0.96) were dissolved in 0.15M pH 7.4 A solution of 20wt% DA-PEG and 3wt% CM-Chitosan was prepared in PBS buffer. Take 0.137g of DA-PEG solution, 1g of CM-Chitosan solution and 0.04g of PBS buffer and mix them uniformly for half a minute, then place them in a constant temperature water bath at 37°C for 0.5-6h to obtain the alkynyl / amino group The molar ratio is 0.28, the CM-Chitosan concentration is 2.5wt% hydrogel, and it does not flow, which is recorded as 0.28-2.5% DA-PEG / CM-Chitosan hydrogel.
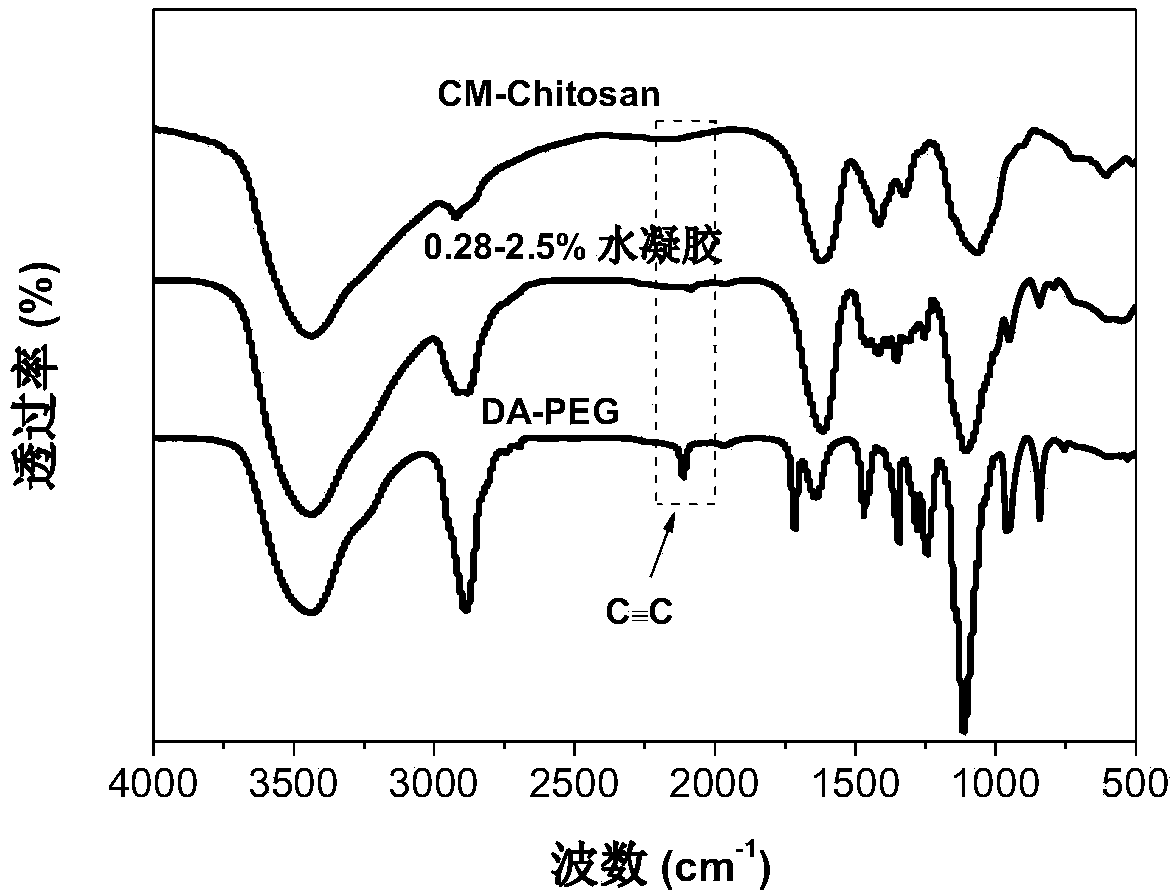
[0057] figure 1 It is the infrared spectrum of 0.28-2.5% DA-PEG / CM-Chitosan hydrogel and its corresponding precursors DA-PEG and CM-Chitosan, from which it can be seen that the alkynyl C of DA-PEG in the hyd...
Embodiment 2
[0062] Preparation of DA-PEG / CM-Chitosan hydrogel by spontaneous amine-alkyne click reaction.
[0063] DA-PEG (PEG molecular weight 6000) and carboxymethyl chitosan (CM-Chitosan, molecular weight 74kDa, deacetylation degree 0.96, carboxymethyl substitution degree 0.96) were dissolved in 0.15M PBS at pH 7.4 In the buffer solution, a solution of 25wt% DA-PEG and 2wt% CM-Chitosan was prepared. Take 0.15g of DA-PEG solution, 1g of CM-Chitosan solution and 0.18g of PBS buffer and mix them uniformly for half a minute, then place them in a constant temperature water bath at 37°C for 8h, and the molar ratio of alkynyl / amino group is 0.2 , 0.2-1.5% DA-PEG / CM-Chitosan hydrogel with a CM-Chitosan concentration of 1.5 wt%. This example demonstrates that varying the molecular weight and concentration of PEG does not affect the preparation of injectable hydrogels. A series of DA-PEG / CM-Chitosan hydrogels can be obtained by changing the molar feed ratio (0.05-5) of the alkynyl group / amino ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| viscosity average molecular weight | aaaaa | aaaaa |
| transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com