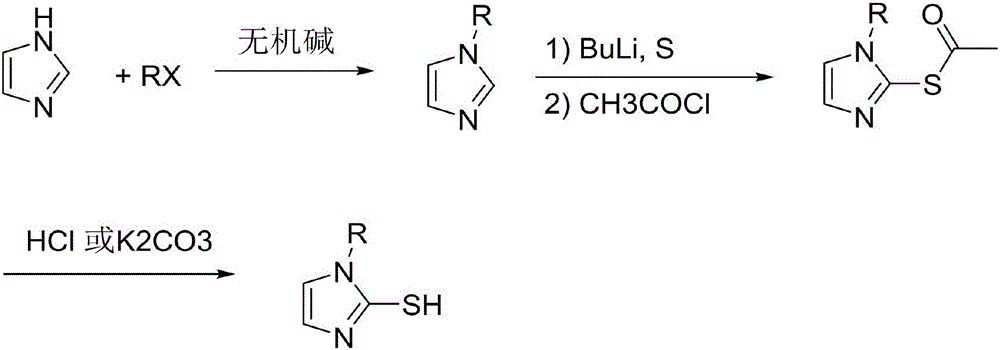
Preparation method of 2-sulfydryl-1-alkyl imidazole
A technology of alkylimidazole and mercapto, which is applied in the field of preparation of 2-mercapto-1-alkylimidazole, can solve problems such as potential safety hazards, spontaneous combustion and oxidation of sulfur powder, and non-parallel reaction yields, so as to avoid by-products, heavy Good performance, avoid spontaneous combustion and oxidation problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of 2-mercapto-1-methylimidazole compound
[0024]
[0025] In the first step, take imidazole (34.0g, 0.5mol), sodium carbonate (106.0g, 1.0mol) and 800ml of acetone in a 1L autoclave, feed in methyl chloride (50.5g, 1.0mol), and stir vigorously at 60°C 12 hours, then down to room temperature, filter, wash the solid with 10% of the original volume of acetone, the organic phase is concentrated under reduced pressure to remove the solvent, and the remaining liquid is subjected to vacuum distillation (50 ~ 52 ° C, 1 torr), to obtain 36.5g of product N- Methylimidazole compound (2), yield 89%, purity 98.6%.
[0026] In the second step, add N-methylimidazole (36.5g, 0.45mol) and 220mL tetrahydrofuran obtained in the previous step into a three-necked reaction flask, then drop the temperature to -15°C and add 196mL (0.49mol) of n-butyllithium-n-hexane solution dropwise. , 2.5moL / L), after the dropwise addition, keep it at -15°C for 2 hours, then add elemental sulf...
Embodiment 2
[0029] Synthesis of 2-mercapto-1-methylimidazole compound
[0030]
[0031] In the first step, take imidazole (34.0g, 0.5mol), potassium carbonate (138.2g, 1.0mol) and 800ml of acetone in a 1L autoclave, feed methyl bromide (57.0g, 0.6mol), and stir vigorously at 60°C for 9 hours, then down to room temperature, filter, wash the solid with 10% of the original volume of acetone, the organic phase is concentrated under reduced pressure to remove the solvent, and the remaining liquid is subjected to vacuum distillation (50~52°C, 1torr), to obtain 37.4g of product N-formazan The imidazole compound (2) has a yield of 91% and a purity of 98.5%.
[0032] In the second step, add N-methylimidazole (37.4g, 0.46mol) and 220ml 2-methyltetrahydrofuran obtained in the previous step into a three-necked reaction flask, then cool down to -15°C and add n-butyllithium-n-hexane solution dropwise 191mL (0.48mol, 2.5moL / L), after the dropwise addition, keep the temperature at -15°C for 2 hours, ...
Embodiment 3
[0035] Synthesis of 2-mercapto-1-ethylimidazole compound
[0036]
[0037] In the first step, take imidazole (34.0g, 0.5mol), cesium carbonate (244.4g, 0.75mol) and 800ml acetone in a 1L autoclave, feed into ethyl chloride (48.4g, 0.75mol), and mechanically Stir for 12 hours, then cool down to room temperature, filter, wash the solid with 10% of the original volume of acetone, concentrate the organic phase under reduced pressure to remove the solvent, and carry out vacuum distillation (55~58°C, 1torr) for the remaining liquid to obtain 44.2g of product N - Ethyl imidazole compound (2), yield 92%, purity 98.8%.
[0038] In the second step, add N-ethylimidazole (44.2g, 0.46mol) obtained in the previous step and 250ml of diethoxymethane into a three-necked reaction flask, then cool down to -15°C and add n-butyllithium-n-hexane solution dropwise 193mL (0.48mol, 2.5moL / L), after the dropwise addition was completed, it was incubated and reacted at -15°C for 2 hours, and then ele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com