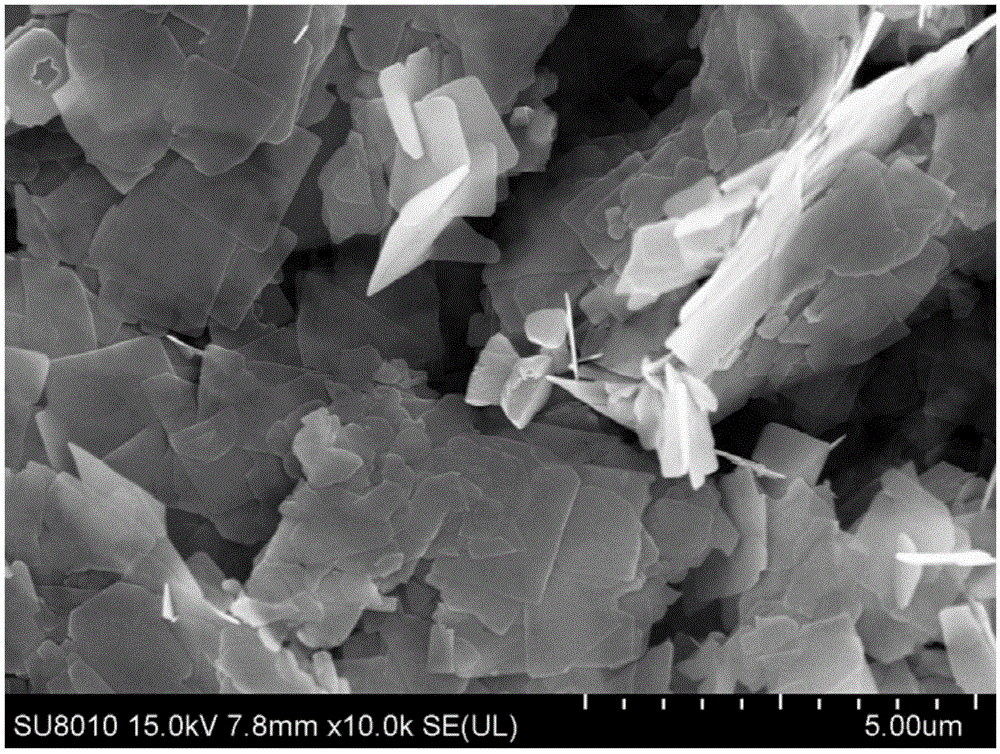
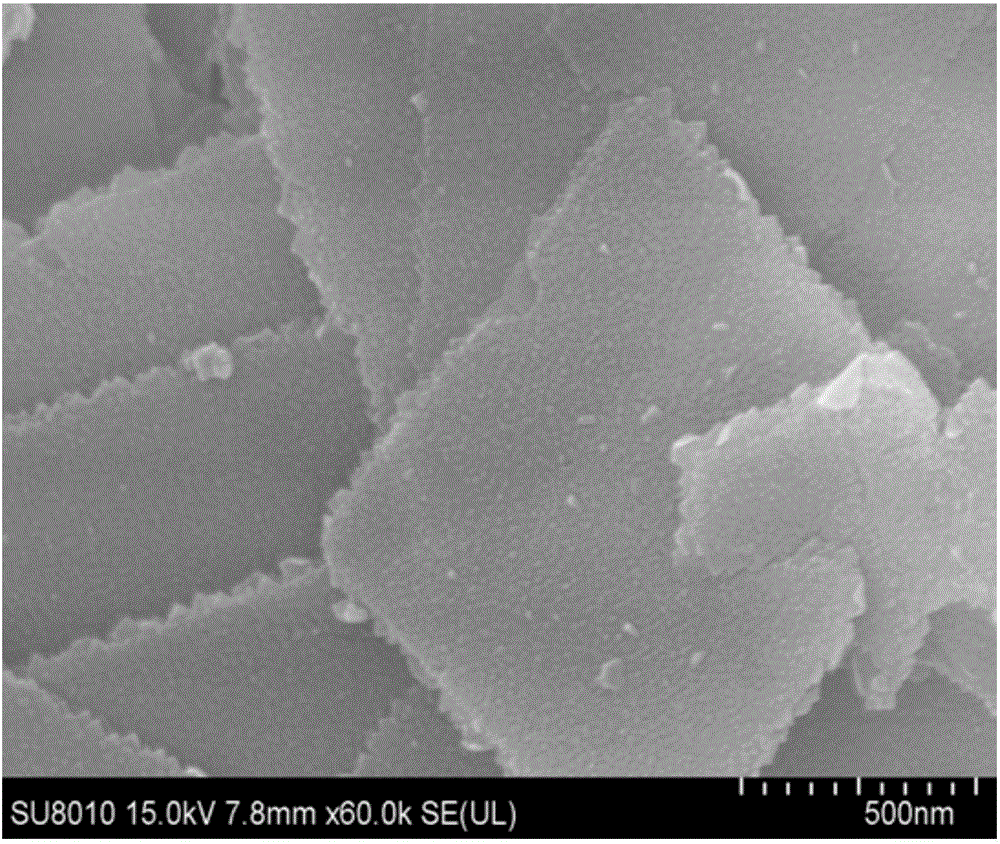
Bismuth-oxybromide-cadmium sulfide nano composite photocatalyst and preparation method thereof
A bismuth oxybromide and nanocomposite technology, which is applied in the field of photocatalyst material preparation, can solve the problems of limiting solar energy utilization, achieve high visible light catalytic activity, simple process equipment, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] ①. Preparation of bismuth oxybromide nanosheets
[0026] Weigh 1.8mmol of CTAB (about 0.6547g), 1.8mmol of Bi(NO 3 ) 3 ·5H 2 O (about 0.8731g) was put into the reaction kettle, added 48mL of distilled water, stirred for 25min to make it fully dissolved, after that, adjusted the pH of the solution to neutral with 1M NaOH solution, continued to stir for 1.5h; kept at 160°C for 30h Afterwards, naturally cool to room temperature, suction filter, dry, and collect square-shaped bismuth oxybromide nanosheets.
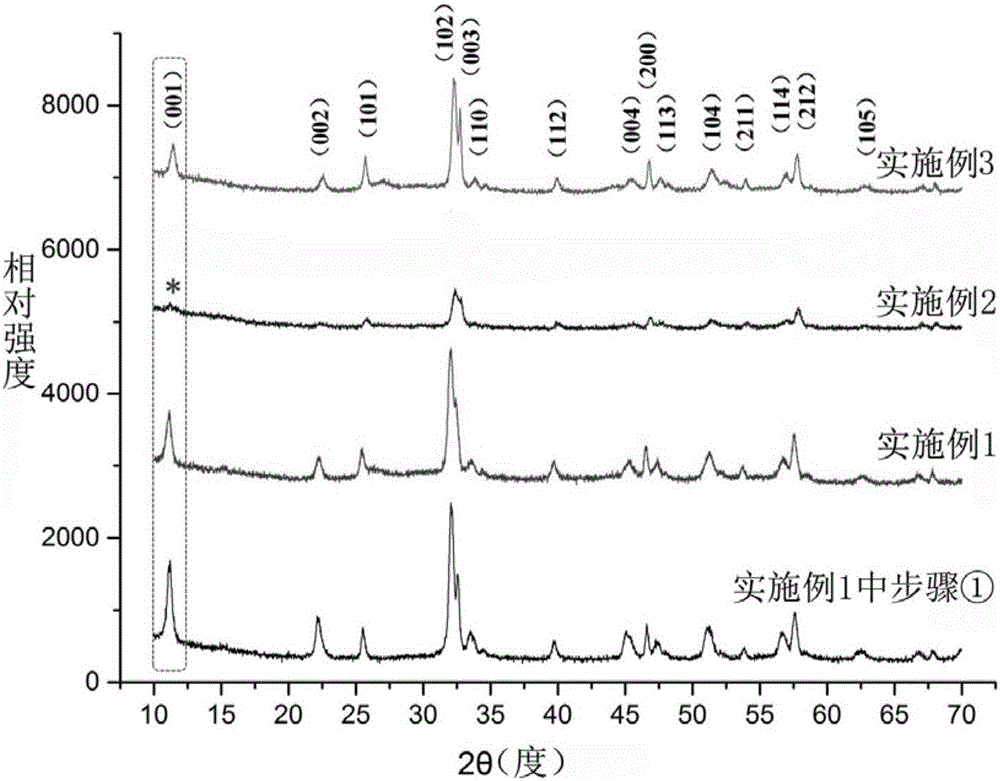
[0027] See attached figure 1 , by the X-ray powder diffraction analysis (XRD) spectrogram of the bismuth oxybromide nanosheet that the method described in step 1. in embodiment 1 makes. The peak positions of the spectral lines in the figure correspond to all the diffraction crystal planes of the JCPDF standard card (09-0393), indicating that they are tetragonal BiOBr crystals, space group P4 / nmm, and lattice constant No impure diffraction peaks were found, indicat...
Embodiment 2
[0034] ①. Preparation of bismuth oxybromide nanosheets
[0035] Weigh 1.2mmol of CTAB (about 0.4365g), 1.2mmol of Bi(NO 3 ) 3 ·5H 2 O (about 0.5820g) is put into the reaction kettle, add 40mL of distilled water, stir for 15min to fully dissolve, then adjust the pH of the solution to neutral with 1M NaOH solution, continue stirring for 0.5h; keep warm at 180°C for 10h Afterwards, naturally cool to room temperature, suction filter, dry, and collect square-shaped bismuth oxybromide nanosheets.
[0036] ②. Reaction of cadmium sulfide nanocrystals on bismuth oxybromide nanosheets
[0037] Take a tall beaker A, add 0.0914g of bismuth oxybromide (about 0.3mmol) nanosheets and 30mL of distilled water, ultrasonically disperse for 18min; then add 0.2mmol of Cd(CH 3 COO) 2 ·3H 2 0, stir and dissolve; get another beaker B, add 1mmol of thiourea (0.0761g), 0.4mL of ethylenediamine and 30mL of water, stir and dissolve to be a transparent homogeneous clear solution; the solution in bea...
Embodiment 3
[0041] ①. Preparation of bismuth oxybromide nanosheets
[0042] Weigh 1.5mmol of CTAB (about 0.5456g), 1.5mmol of Bi(NO 3 ) 3 ·5H 2 O (about 0.7276g) is put into the reaction kettle, add 45mL of distilled water, stir for 20min to make it fully dissolved, then adjust the pH of the solution to neutral with 1M NaOH solution, and continue to stir for 1h; after 17h at 170°C , naturally cooled to room temperature, suction filtered, dried, and collected to obtain square bismuth oxybromide nanosheets.
[0043] ②. Reaction of cadmium sulfide nanocrystals on bismuth oxybromide nanosheets
[0044] Take a tall beaker A, add 0.1000 g of bismuth oxybromide (about 0.328 mmol) nanosheets and 30 mL of distilled water, and ultrasonically disperse for 20 min; then add 0.4 mmol of Cd(CH 3 COO) 2 ·3H 2 0, stir and dissolve; get another beaker B, add 1mmol of thiourea (0.0761g), 0.4mL of ethylenediamine and 30mL of water, stir and dissolve to be a transparent homogeneous clear solution; the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com