Cracked-ring lupane derivatives and pharmaceutical application thereof
A technology of lupine and its derivatives, which is applied in the direction of androstane derivatives, drug combinations, antipyretics, etc., and can solve the problems of high incidence of thrombotic diseases and high clinical costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
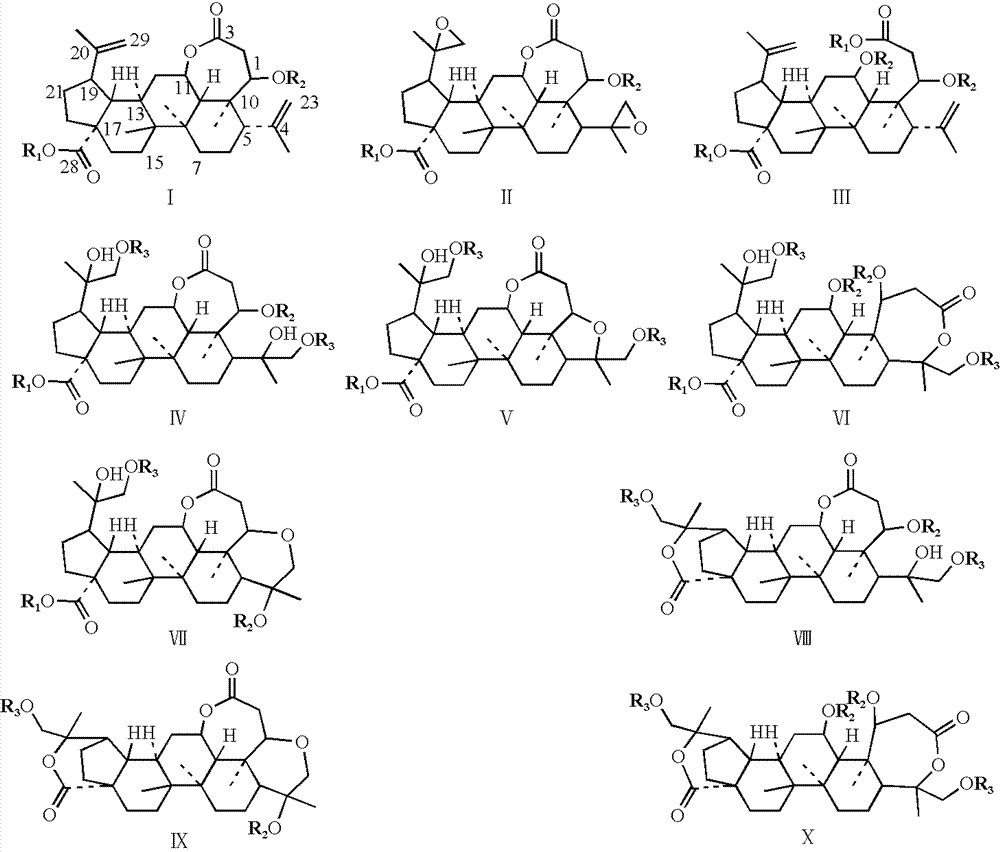
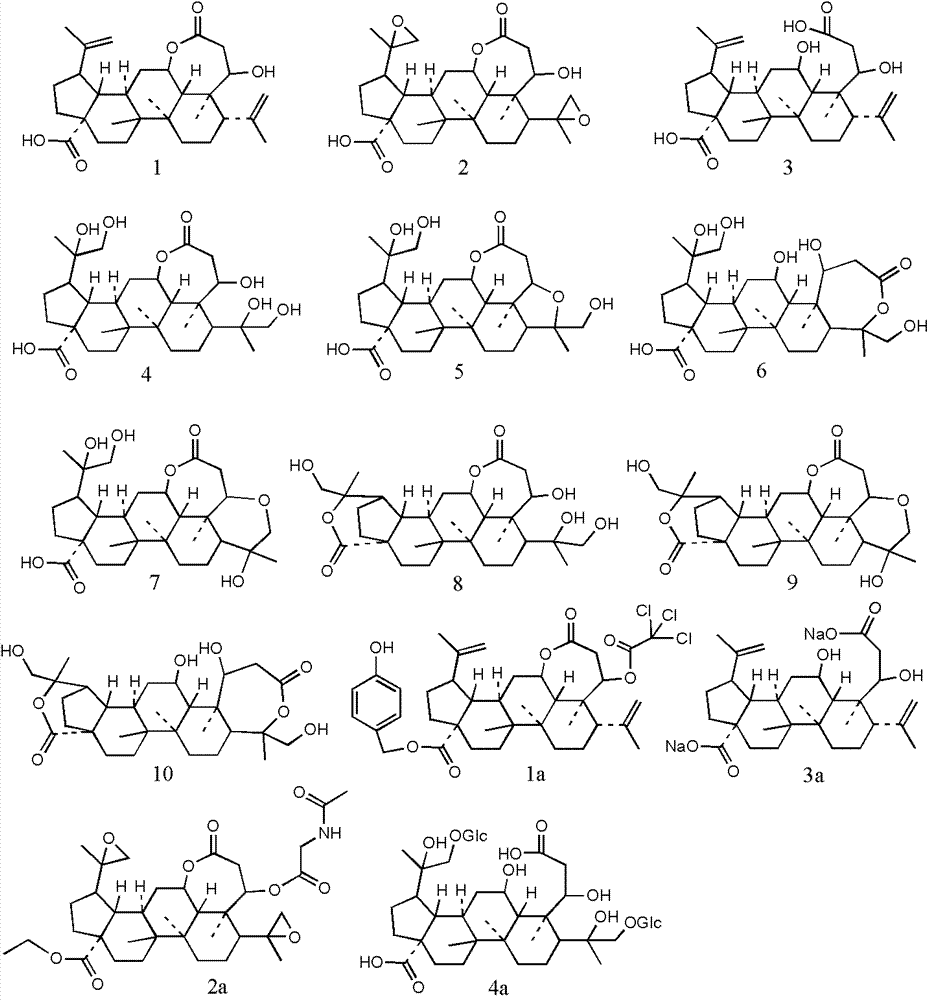
Image
Examples
Embodiment 1
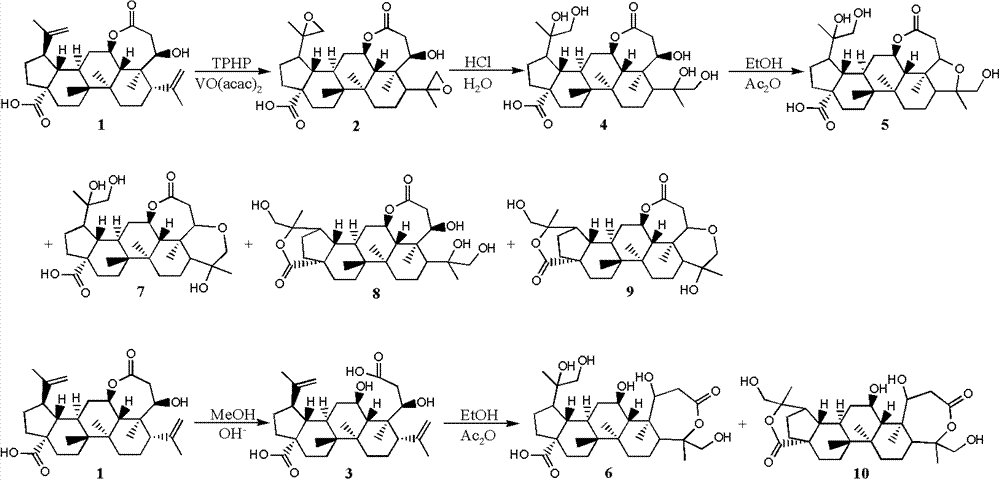
[0026] Example 1: Synthesis of split ring lupin derivatives
[0027] 1.1 Instruments and reagents
[0028] 1 HNMR nuclear magnetic resonance spectrum adopts Bruker AVII 500 superconducting nuclear magnetic resonance instrument; HRMS mass spectrometry adopts UPLC-XevoG 2 -S QTOF-MS / MS. Derivative 1 is self-made in the laboratory (purity ≥ 98%), and other reagents are domestic analytical reagents.
[0029] 1.2 Synthesis of derivatives
[0030] 1.2.1 Synthesis of Derivative 2
[0031] Dissolve derivative 1 (484.0mg, 1.0mmol) in 50mL of dichloromethane, add vanadyl acetylacetonate (26.0mg, 0.1mmol), stir well and add 70% tert-butyl hydroperoxide aqueous solution (413μL, 3mmol) , react at room temperature for 3 hours, add 30mL aqueous sodium bicarbonate solution, stir for 15 minutes, extract 3 times with ethyl acetate 30mL, combine the organic layers, wash with saturated brine, dry over anhydrous sodium sulfate, and recover the solvent under reduced pressure to obtain derivati...
Embodiment 2
[0063] Example 2: P2Y12 receptor antagonist effect test of split-lupene derivatives
[0064] 2.1 Method: Blood was collected from healthy volunteers using a 20 mL syringe containing 2 mL of buffered sodium citrate. Blood was transferred to polypropylene tubes and centrifuged (100 g) for 5 min at room temperature. The platelet-rich plasma (PRP) floating on the surface was then collected, diluted, and platelet counts were performed before it was used for aggregation measurements.
[0065] Measurements of platelet aggregation (Aggregometer) were performed in glass tubes at 37°C. 4 μL of the test compound (DMSO solution 100-fold higher than the desired final concentration) was mixed with 392 μL of freshly prepared PRP and incubated for 1 min with stirring. Then add 4 μL of 250 μM ADP solution to the mixture. Stirring was continued and aggregation measurements were monitored for 6-8 min by recording the change in optical density according to the method of G.V.R. Born (Born. Natu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com