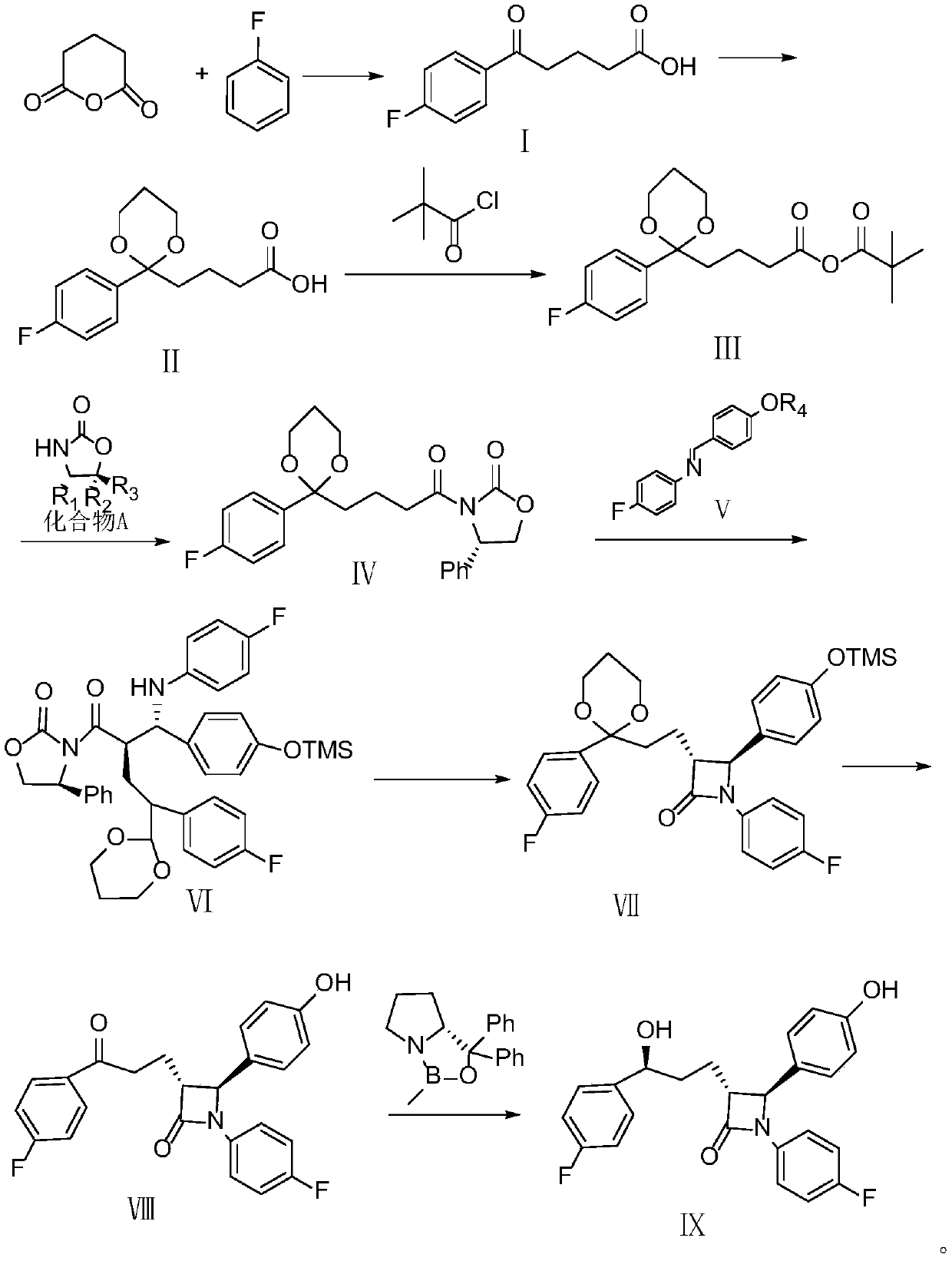
A kind of ezetimibe intermediate compound
A technology of ezetimibe and intermediates, which is applied in the field of ezetimibe intermediate compounds, can solve the problems of incomplete reaction, unfavorable industrialized large-scale production, large impurities, etc., and achieve the effect of simple production and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] step 1:
[0067] Measure 180ml of tetrahydrofuran and dichloromethane mixed solvent (the ratio of the two is 1:3) into a 500ml round bottom flask, add 0.8gZnCl 2 And 2ml HCl, add 32ml trimethylorthoformate, react 4-(4-fluoro-benzoyl)-butyric acid (II) with ethanedithiol at 20°C for 4 hours. Add 5g of sodium bicarbonate to terminate the reaction. The solvent was evaporated, the residue was dissolved in 150ml of methanol, and cooled in an ice-water bath. During the cooling process, 100ml of 10% potassium hydroxide solution was added, the temperature was controlled at 25°C, and stirred for 30 minutes. Concentrate, add 300ml 10% tartaric acid solution to make the pH reach 3-4, extract twice with 200ml+100ml ethyl acetate, wash the organic phase with water until neutral, dry over anhydrous sodium sulfate, filter, evaporate to dryness, and recrystallize from n-hexane to obtain 4-[2-(4-Fluoro-phenyl)-[1,3]dithiolan-2-yl]-butyryl-4-phenyl-oxazolidin-2-one (IV). Yield 94.6%. ...
Embodiment 2
[0077] step 1:
[0078] Measure 180ml of tetrahydrofuran and dichloromethane mixed solvent (the ratio of the two is 1:3) into a 500ml round bottom flask, add 0.8gZnCl 2 And 2ml HCl, add 32ml trimethylorthoformate, react 4-(4-fluoro-benzoyl)-butyric acid (II) with ethanedithiol at 20°C for 4 hours. Add 5g of sodium bicarbonate to terminate the reaction. The solvent was evaporated, the residue was dissolved in 150ml of methanol, and cooled in an ice-water bath. During the cooling process, 100ml of 20% sodium hydroxide solution was added, the temperature was controlled at 25°C, and stirred for 30 minutes. Concentrate, add 300ml 10% tartaric acid solution to make the pH reach 3~4, extract twice with 200ml+100ml dichloromethane, wash the organic phase with water until neutral, dry over anhydrous sodium sulfate, filter, evaporate to dryness, and recrystallize from n-hexane Obtain 4-[2-(4-fluoro-phenyl)-[1,3]dithiolan-2-yl]-butyryl-4-phenyl-oxazolidin-2-one (IV) . Yield 95.5%.
...
Embodiment 3
[0088] step 1:
[0089] Measure 180ml tetrahydrofuran into a 500ml round bottom flask, add 0.8g ZnCl 2 And 2ml HCl, add 32ml trimethylorthoformate, react 4-(4-fluoro-benzoyl)-butyric acid (II) with ethanedithiol at 20°C for 4 hours. Add 5g of sodium bicarbonate to terminate the reaction. The solvent was evaporated, the residue was dissolved in 150ml of methanol, and cooled in an ice-water bath. During the cooling process, 100ml of 30% lithium hydroxide solution was added, the temperature was controlled at 25°C, and stirred for 30 minutes. Concentrate, add 300ml of 10% tartaric acid solution to make the pH reach 3-4, extract twice with 200ml + 100ml of n-hexane, wash the organic phase with water until neutral, dry over anhydrous sodium sulfate, filter, evaporate to dryness, and recrystallize from n-hexane to obtain 4 -[2-(4-Fluoro-phenyl)-[1,3]dithiolan-2-yl]-butyryl-4-phenyl-oxazolidin-2-one (IV). Yield 93.9%.
[0090] Step 2:
[0091] In an anhydrous and oxygen-free envi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com