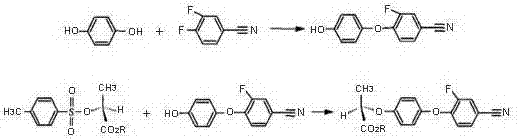
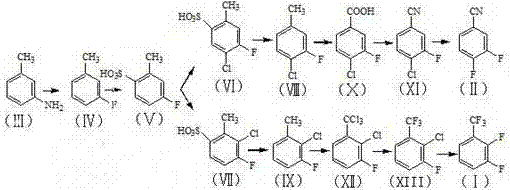
Continuous production method for industrially preparing 2,3-difluorobenzotrifluoride and 3,4-difluorobenzonitrile
A technology of difluorotrifluorotoluene and difluorobenzonitrile, which is applied in chemical instruments and methods, preparation of carboxylic acids by ozone oxidation, preparation of organic compounds, etc., can solve the problems of high cost, incomplete reaction, and high cost of raw materials, and achieve Low cost, high production efficiency, and the effect of improving product efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Preparation of compound of formula (IV) (m-fluorotoluene) 1 by diazotization reaction:
[0074] Put 1236g (61.8mol) of hydrogen fluoride into a 5L fluorination reactor and cool down to 0°C, add 110g dropwise per hour and add 2153g (20.1mol) of m-methylaniline; stir and mix for 3 hours and cool and keep at -5°C, add every hour Add 1526g sodium nitrite to 292g, react at low temperature for 1h, slowly raise the temperature to 35°C at 4°C / h and react for 3h, the content of m-methylaniline should be less than 1wt%, let stand and separate, collect the organic phase, a total of 2025g, to obtain m-fluorotoluene, purity The yield was 98.4%, and the yield was 90.1%; hydrogen fluoride was recovered by condensation at -5°C.
Embodiment 2
[0075] Example 2: Preparation of compound of formula (IV) (m-fluorotoluene) 2 by diazotization reaction:
[0076] Put 1,497g (74.9mol) of hydrogen fluoride into a 5L fluorination reactor and cool down to 0°C, add 110g dropwise per hour, add 2,013g (18.8mol) of m-methylaniline; stir and mix for 3 hours, cool and keep at -5°C, and put in every hour Add 1548g sodium nitrite to 290g, react at low temperature for 1h, slowly raise the temperature to 35°C at 4°C / h and react for 3h. The yield was 97.7%, and the yield was 86.3%; hydrogen fluoride was recovered by condensation at -5°C.
Embodiment 3
[0077] Example 3: Preparation of compound of formula (V) (2-methyl-4-fluoro-benzenesulfonic acid) by substitution of sulfonic acid group:
[0078] Put 1258g of 95% sulfuric acid and 122g of sulfur trioxide into a reactor with stirring, temperature control and reflux, add 1120g (10.2mol) of m-fluorotoluene with a purity of 99%; heat to 30°C for 6h, and collect the sulfonic acid phase; The products are 1706g (about 88%) of 2-methyl-4-fluoro-benzenesulfonic acid and 182g (about 9.4%) of 2-fluoro-4-methylbenzenesulfonic acid; put them into 6.7L of toluene, stir and heat up to After dissolving at 65°C, maintain it for 2h, cool down naturally to 25°C for 6h to crystallize, and filter to obtain 947g of 4-fluoro-2-methylbenzenesulfonic acid with a purity of 98%. The toluene filtrate is recovered and recycled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com