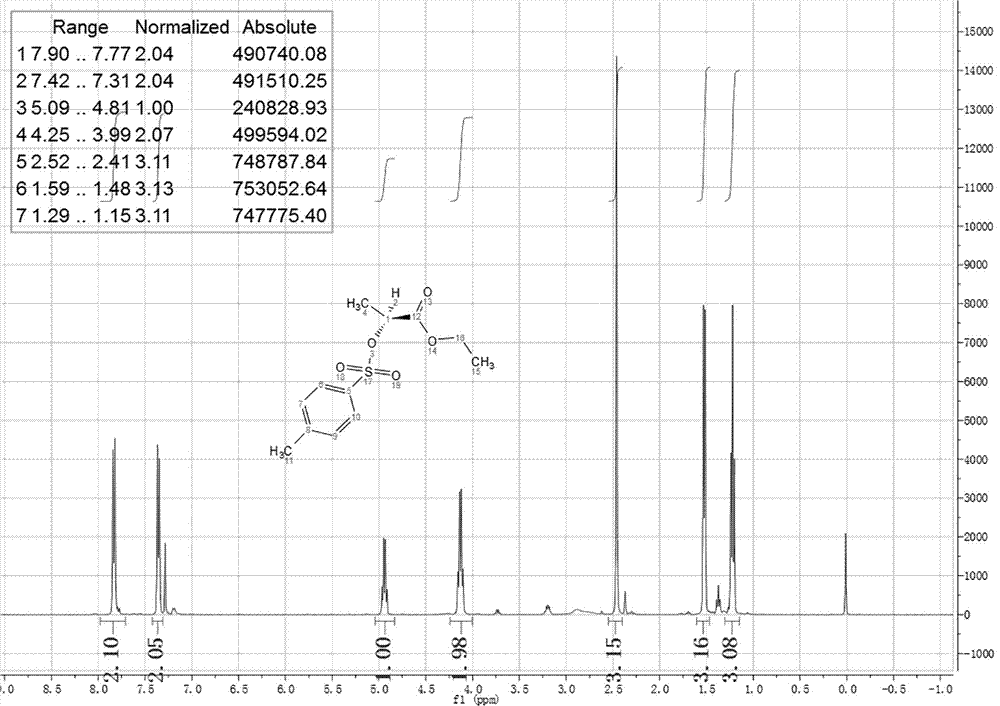
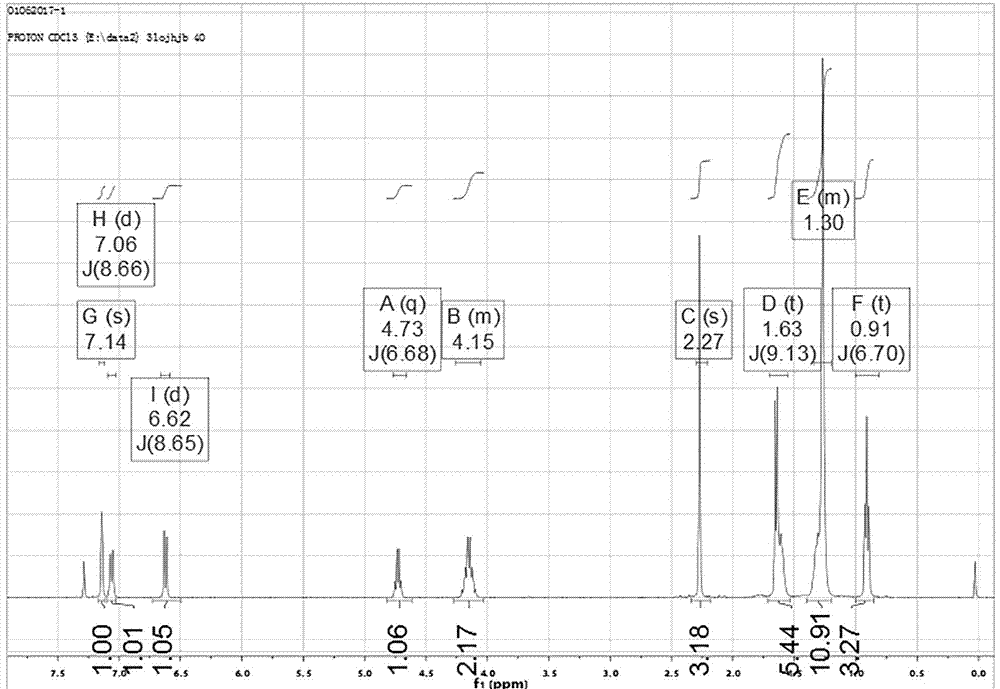
Preparation method of octyl (R)-2-(4-chloro-2-methylphenoxy) propionate root retarder
A technology of ethyl toluenesulfonylpropionate and methylphenoxy, which is applied in the field of preparation of octyl-2-propionate root inhibitor, and achieves the effects of high yield, mild reaction conditions and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of (R)-2-(4-chloro-2-methylphenoxy) octyl propionate root inhibitor, comprising the following preparation steps:
[0030] The synthesis of S1.(S)-2-ethyl p-toluenesulfonyl propionate, concrete steps are as follows:
[0031] Add 118.0 g (1.0 mol) of L-ethyl lactate, 191.0 g (1.0 mol) of p-toluenesulfonyl chloride and 700 mL of toluene into a 2L three-neck round bottom flask equipped with a mechanical stirring device, stir at room temperature to dissolve all the solids and mix well , began to add 168.0 mL (1.2 mol) of triethylamine dropwise, and the drop was completed within 1 h. Continue the mechanical stirring reaction at 28°C to 35°C, monitor the reaction process by TLC, add water to the reaction system and continue stirring for half an hour after the raw materials have reacted, separate the liquid with a separatory funnel, and backwash the water phase twice with dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfat...
Embodiment 2
[0037] The difference between this embodiment and Example 1 is that the specific steps for the synthesis of S1. (S)-2-ethyl p-toluenesulfonylpropionate are: add L- 29.5g (0.25mol) of ethyl lactate, 47.8g (0.25mol) of p-toluenesulfonyl chloride and 175mL of toluene were stirred at room temperature to dissolve all the solids and mix evenly. Start to add 0.275mol of pyridine dropwise and finish the dropwise within 0.5h. Continue the mechanical stirring reaction at 28°C to 35°C, monitor the reaction process by TLC, add water to the reaction system and continue stirring for half an hour after the raw materials have reacted, separate the liquid with a separatory funnel, and backwash the water phase twice with dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated, and 60.6 g of light yellow transparent oily product (S)-2-ethyl p-toluenesulfonylpropionate was obtained after vacuum filtration. Others are the...
Embodiment 3
[0039] The difference between this embodiment and Example 1 is that the specific steps for the synthesis of S1. (S)-2-ethyl p-toluenesulfonylpropionate are: add L- Ethyl lactate 29.5g (0.25mol), p-toluenesulfonyl chloride 47.8g (0.25mol) and 175mL toluene, stir at room temperature to dissolve all the solids and mix evenly, start to drop 0.275mol of 4-dimethylaminopyridine, within 0.5h Drip finished. Continue the mechanical stirring reaction at 28°C to 35°C, monitor the reaction process by TLC, add water to the reaction system and continue stirring for half an hour after the raw materials have reacted, separate the liquid with a separatory funnel, and backwash the water phase twice with dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, and filtered. The filtrate was concentrated, and 62.0 g of light yellow transparent oily product (S)-2-ethyl p-toluenesulfonylpropionate was obtained after vacuum filtration. Others are the same as embodime...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com