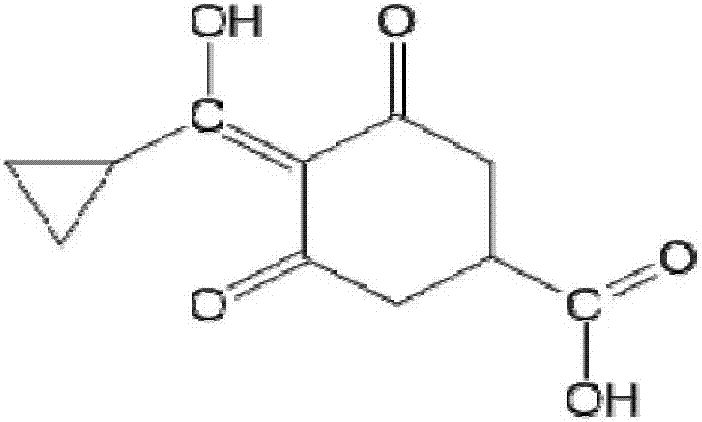
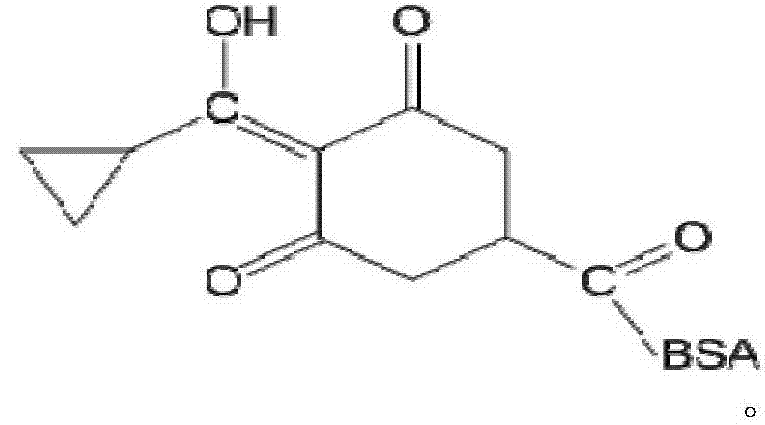
Trinexapac-ethyl artificial antigens, a preparing method of a specific antibody and uses of the antibody
A technology for trinexapac-ethyl and hapten, which is applied in the field of immunochemical analysis to achieve the effects of low cost, convenient on-site monitoring and strong antibody specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1. Synthesis of artificial haptens
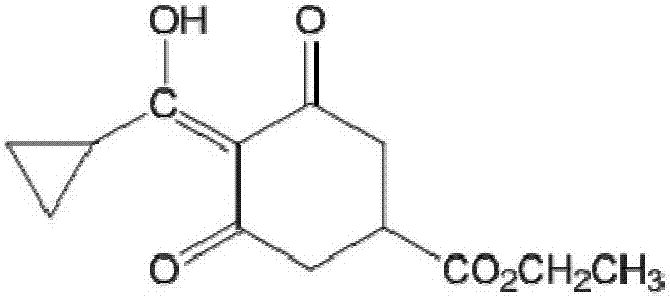
[0035] Carboxylate on the six-membered ring of trinexapac-ethyl is subjected to alkaline hydrolysis to synthesize a hapten, which not only retains the characteristic group of trinexapac-ethyl to the greatest extent, but also forms a carboxyl terminal group, which can be better coupled with the protein. After purification, the product was identified by mass spectrometry (ESI) and hydrogen nuclear magnetic resonance spectrum (1H-NMR). The molecular structural formula of trinexapac-ethyl artificial hapten is:
[0036]
[0037] The specific preparation method is as follows: Weigh 5.04g (about 0.02mol) of trinexapac-ethyl and 30mL of tetrahydrofuran into a 100mL conical flask, then add 24mL of 5% sodium hydroxide methanol solution (trinexapac-ethyl: sodium hydroxide=1: 1.5 mol ratio), stirred at room temperature for 4 h and added 20 mL of water; the mixed solution was extracted with ethyl acetate, the organic phase was discarded, the ...
Embodiment 2
[0039] Identical to the preparation method of Example 1, the difference is that the proportioning of each raw material is different:
[0040] The specific preparation method is as follows: Weigh 7.56g (about 0.03mol) of trinexapac-ethyl and 40mL of tetrahydrofuran into a 100mL conical flask, then add 36mL of 5% sodium hydroxide methanol solution (trinexapac-ethyl: sodium hydroxide=1: 1.5 mol ratio), stirred and reacted at room temperature for 4 h and added 30 mL of water; the mixed solution was extracted with ethyl acetate, the organic phase was discarded, the aqueous phase was adjusted to pH 2.0 with concentrated hydrochloric acid, extracted with ethyl acetate, and the organic phases were combined, without After drying over sodium sulfate, it was concentrated under reduced pressure to obtain a yellow oil; it was purified with a silica gel column, and the eluent was a mixture of 150 mL of methanol:dichloromethane=1:40 (volume ratio), and the fractions were collected and concent...
Embodiment 3
[0042] Identical to the preparation method of Example 1, the difference is that the proportioning of each raw material is different:
[0043]The specific preparation method is as follows: Weigh 10.08g (about 0.04mol) of trinexapac-ethyl and 60mL of tetrahydrofuran into a 100mL conical flask, then add 48mL of 5% sodium hydroxide methanol solution (trinexapac-ethyl: sodium hydroxide=1: 1.5 molar ratio), stirred at room temperature for 5 h and added 40 mL of water; the mixed solution was extracted with ethyl acetate, the organic phase was discarded, the aqueous phase was adjusted to pH 2.0 with concentrated hydrochloric acid, extracted with ethyl acetate, and the organic phases were combined, without After drying with sodium sulfate, it was concentrated under reduced pressure to obtain a yellow oil; it was purified with a silica gel column, and the eluent was a mixture of 110 mL of methanol:dichloromethane=1:50 (volume ratio), and the fractions were collected and concentrated to n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com