Preparation method of sulfone compound
A technology of compounds and sulfones, which is applied in the field of preparation of sulfones, can solve problems such as limiting synthetic applications, achieve broad industrial application value, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of a kind of sulfone compound comprises the following steps:
[0027] a) dispersing the sulfonyl hydrazide with structure (I) in the cycloalkane solvent with structure (II);
[0028]
[0029] b) adding an iron salt catalyst and a peroxide oxidizing agent to the mixture obtained in step a), and reacting at a temperature of 90-130° C. for 6-12 hours to obtain a sulfone compound containing structure (III);
[0030]
[0031] When the R is an aryl group, the aryl group is phenyl or naphthyl;
[0032] When the R is a substituted aryl group, the substituted aryl group is p-chlorophenyl, p-tolyl, 2,4,6-trimethylphenyl, p-methoxyphenyl, m-methoxyphenyl , o-methoxyphenyl, p-trifluoromethylphenyl, p-tert-butylphenyl, p-fluorophenyl, p-iodophenyl, 3-nitro-4-chlorophenyl, 4-methyl-3 - fluorophenyl, p-acetamidophenyl or p-bromophenyl;
[0033] The said n is 1, 2, 3 or 4;
[0034] The iron salt is ferric chloride, ferrous sulfate, ferric oxide, ferric ...
Embodiment 1
[0042] In a clean and dry 10 ml Schlenk reaction tube, add 0.50 mmol p-toluenesulfonyl hydrazide, 1 ml cyclohexane, 0.025 mmol ferric chloride, and 1 mmol di-tert-butyl peroxide successively, and the reaction tube Seal and react at 120°C for 6 hours. After the reaction, the reaction mixture was directly spin-dried by a rotary evaporator to dry the cyclohexane in the reaction, and the remaining residue was separated by a silica gel column with petroleum ether as an eluent. , carbon spectrum and high-resolution mass spectrometry analysis confirmed that the target product was p-toluenecyclohexyl sulfone, and the yield was 80%.
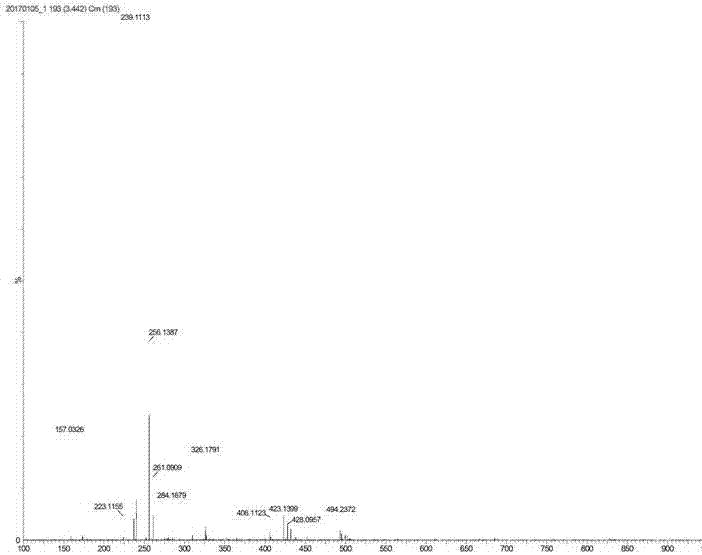
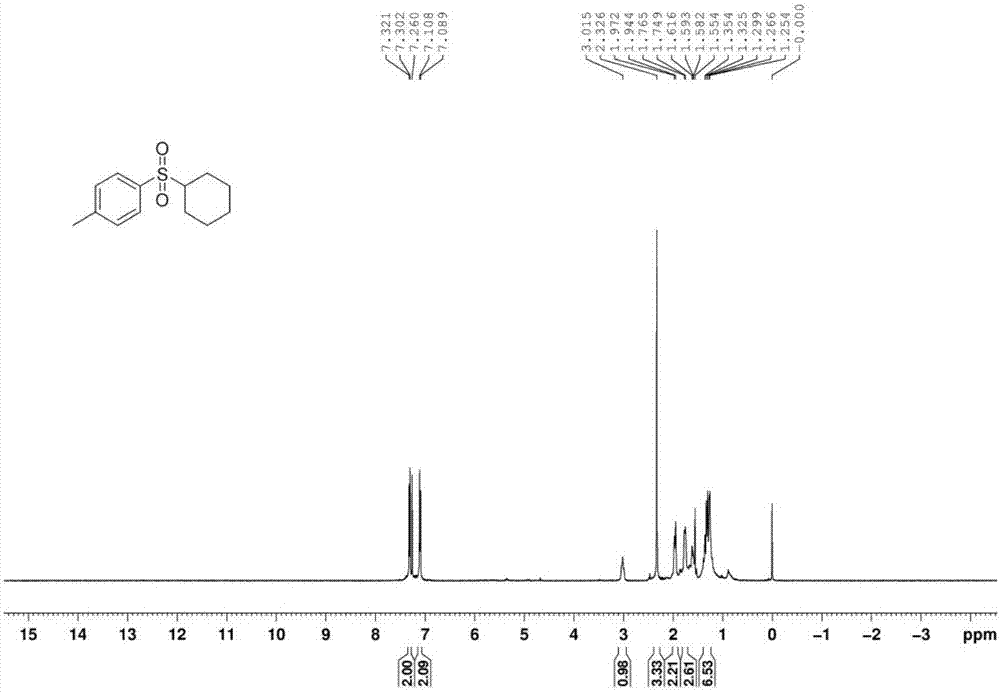
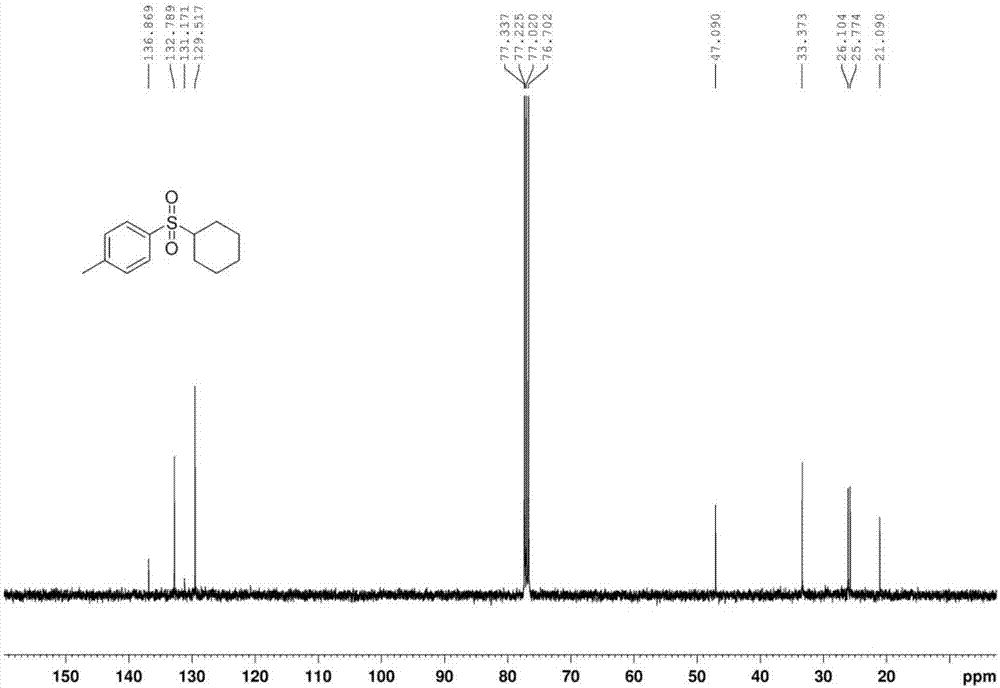
[0043] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 1a As shown, the carbon NMR spectrum is shown as Figure 1b As shown, the high-resolution mass spectrum is shown as Figure 1c shown.
Embodiment 2
[0045] In a clean and dry 10 ml Schlenk reaction tube, add 0.50 mmol p-toluenesulfonyl hydrazide, 1 ml cyclohexane, 0.03 mmol iron sulfate, and 1 mmol di-tert-butyl peroxide in sequence, and seal the reaction tube , reacted at 130°C for 10 hours. After the reaction, the reaction mixture was directly spin-dried by a rotary evaporator to dry the cyclohexane in the reaction, and the remaining residue was separated by a silica gel column with petroleum ether as an eluent. , carbon spectrum and high-resolution mass spectrometry analysis confirmed that the target product was p-toluenecyclohexyl sulfone, and the yield was 68%.
[0046] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 1a As shown, the carbon NMR spectrum is shown as Figure 1b As shown, the high-resolution mass spectrum is shown as Figure 1c shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com