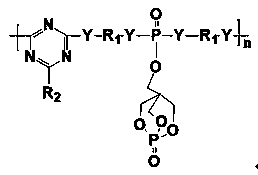
Phosphorus-nitrogen-silicon intumescent flame retardant containing triazine ring and cage structure and its synthesis method
An intumescent flame retardant and a synthesis method technology, which is applied in the field of environment-friendly halogen-free flame retardant products, can solve the problems of insufficient proportion, char-forming performance and flame-retardant performance need to be further improved, and achieve reasonable proportion and good char-forming performance. ability, the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis steps of the phosphorus-nitrogen-silicon intumescent flame retardant containing triazine ring and cage structure are as follows:
[0033] step one
[0034] Add 250mL of acetonitrile to 1000mL, cool to -10°C, add 153.33g (ie 1mol) of phosphorus oxychloride (POCl3) to stir and disperse, and gradually add dropwise 180g (ie 1mol) of 1-oxyphospha-4- Hydroxymethyl-2,6,7-trioxabicyclo[2.2.2]octane (PEPA) and 101.20g (1mol) of triethylamine were reacted for 4 hours to obtain a monosubstituent containing PEPA (POCl2-PEPA );
[0035] step two
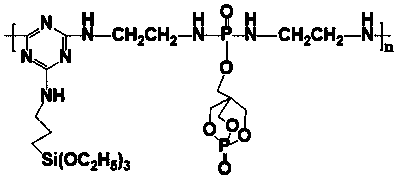
[0036] In another container, add 120.20g (2mol) of ethylenediamine, 50mL of acetonitrile and 202.40g (2mol) of triethylamine and mix them, cool to -10°C, and slowly add 296.83g (1mol) of PEPA-containing The monosubstituent (POCl2-PEPA) was reacted for 6 hours, and the phosphorus-containing diamine intermediate was isolated;
[0037] step three
[0038] In a 1000mL three-necked flask equipped with a reflux condenser, a st...
Embodiment 2
[0044] The synthesis steps of the phosphorus-nitrogen-silicon intumescent flame retardant containing triazine ring and cage structure are as follows:
[0045] step one
[0046] Add 300mL of acetonitrile to 1000mL, cool to 10°C, add 153.33g (1mol) phosphorus oxychloride (POCl3) to stir and disperse, gradually add dropwise 1-oxyphospha-4-hydroxymethyl-2,6,7 -Acetonitrile solution of 180g (1mol) of trioxabicyclo[2.2.2]octane (PEPA) and 101.20g (1mol) of triethylamine, reacted for 1 hour to obtain a monosubstituent containing PEPA (POCl2-PEPA);
[0047] step two
[0048] Add 176.30 g (2 mol) of butanediamine, acetonitrile (50 mL), and 202.40 g (2 mol) of triethylamine into another container, cool to 10°C, and slowly add 296.83 g (1 mol) of The monosubstituent of PEPA (POCl2-PEPA), reacted for 3 hours, and isolated the phosphorus-containing diamine intermediate;
[0049] step three
[0050] In a 1000mL three-necked flask equipped with a reflux condenser, a stirrer, and a consta...
Embodiment 3
[0056] The synthesis steps of the phosphorus-nitrogen-silicon intumescent flame retardant containing triazine ring and cage structure are as follows:
[0057] step one
[0058] Add 300mL of acetonitrile to 1000mL, cool to 0°C, add 153.33g (1mol) of phosphorus oxychloride (POCl3) to stir and disperse, and gradually add 180g (1mol) of 1-oxyphospha-4-hydroxymethyl -2,6,7-trioxabicyclo[2.2.2]octane (PEPA) and 101.20g (1mol) of triethylamine were reacted for 2.5 hours to obtain a monosubstituent containing PEPA (POCl2-PEPA);
[0059] step two
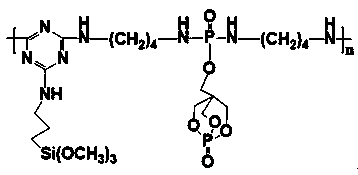
[0060] In another container, add 120.20g (2mol) of ethylenediamine, 50mL of acetonitrile, and 202.40g (2mol) of triethylamine and mix them, cool to 0°C, and slowly add 296.83g (1mol) of ), the monosubstituent of PEPA (POCl2-PEPA), reacted for 4.5 hours, and isolated the phosphorus-containing diamine intermediate;
[0061] step three
[0062] In a 1000mL three-necked flask equipped with a reflux condenser, a stirrer, and a constant pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com