Equipment that can directly produce hydrogen by using salt difference and its application method
A salt difference energy and direct technology, applied in the direction of electrolysis components, electrolysis process, diaphragm, etc., can solve the problems of high energy consumption, harsh selection of metal electrodes, high energy consumption of hydrolysis, etc., to reduce energy consumption, improve electrode stability, and produce The effect of high hydrogen efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
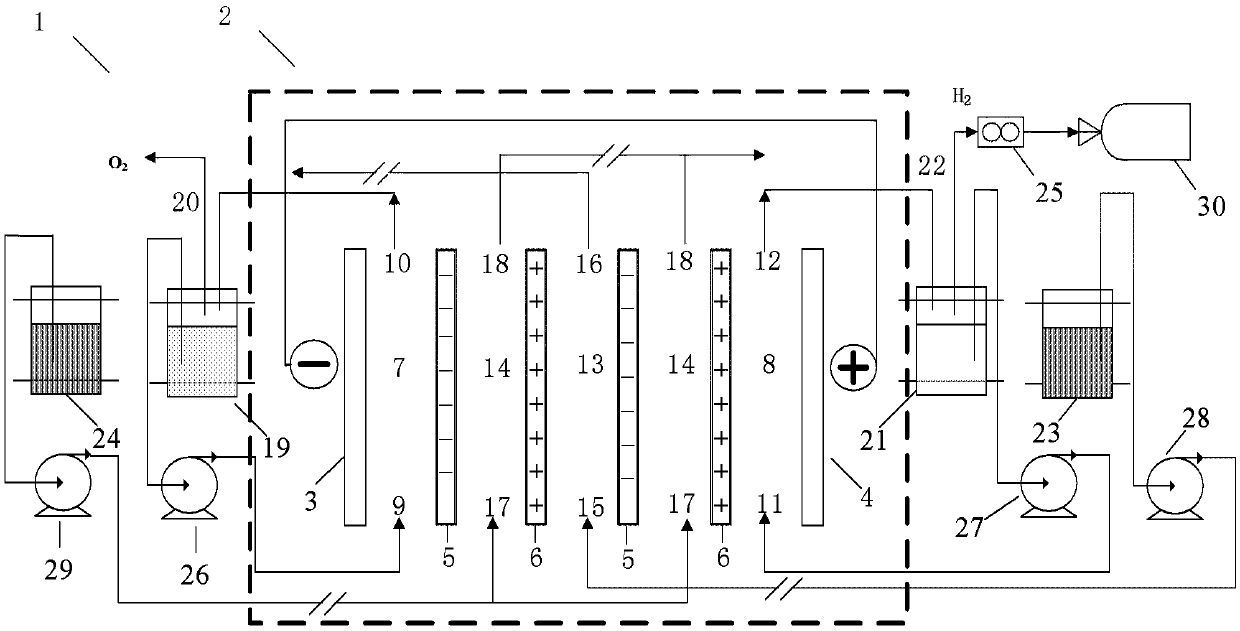
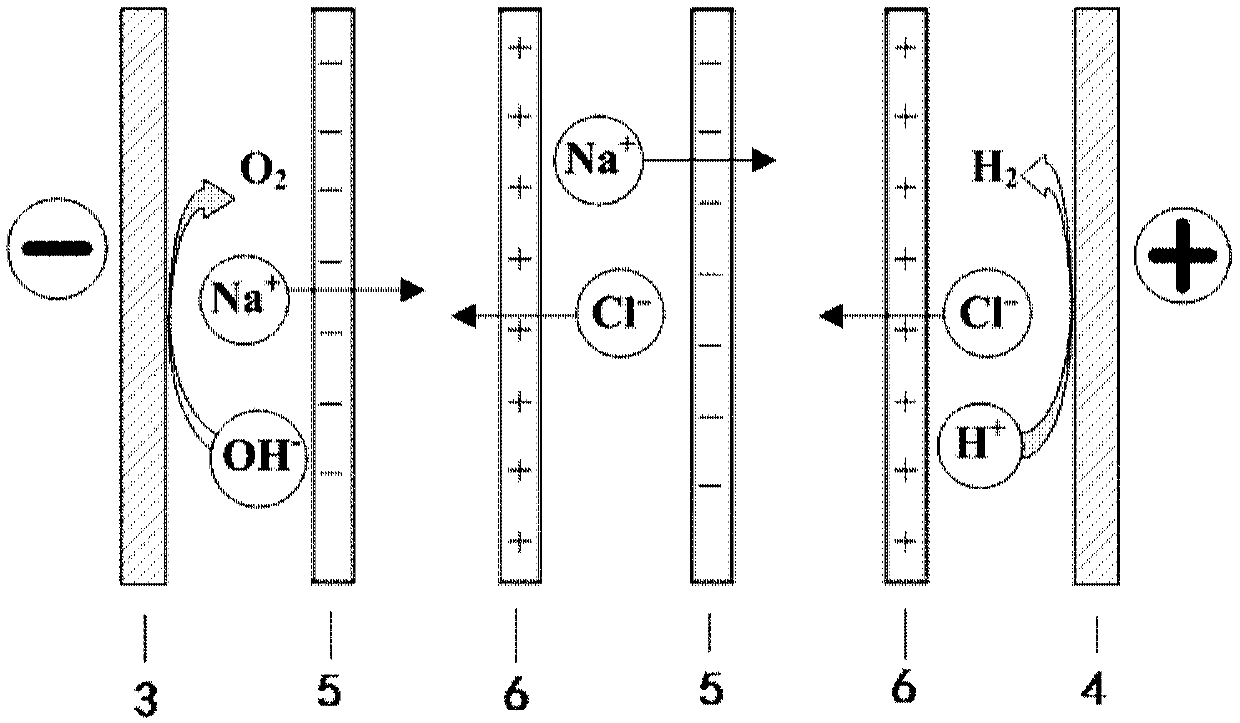
[0070] This example uses figure 1 The equipment shown in the figure can directly produce hydrogen by using the salt difference. The concentrated solution used is an aqueous sodium chloride solution with a concentration of 4.0mol / L, the light solution used is an aqueous sodium chloride solution with a concentration of 0.017mol / L, and the lye used is an aqueous sodium hydroxide solution with a concentration of 0.5mol / L , and the acid solution used is an aqueous hydrochloric acid solution with a concentration of 2.0 mol / L.
[0071] Construction basis figure 1 The equipment that can directly produce hydrogen by utilizing the salt difference shown in , in which 20 cation exchange membranes and 20 anion exchange membranes are used in total, and 19 concentrated solution chambers and 20 dilute solution chambers are formed in the reverse electrodialysis device combination unit. The anode and cathode plates used are ruthenium-coated titanium plates produced by Baoji Qixin Titanium In...
Embodiment 2
[0081] In a similar manner to Example 1 by figure 1 The equipment that can directly produce hydrogen by using the salt difference shown in , the difference is that the concentration of hydrochloric acid in the acid solution is changed to 1.0mol / L, and the concentration of sodium hydroxide in the lye is changed to 0.5 mol / L.
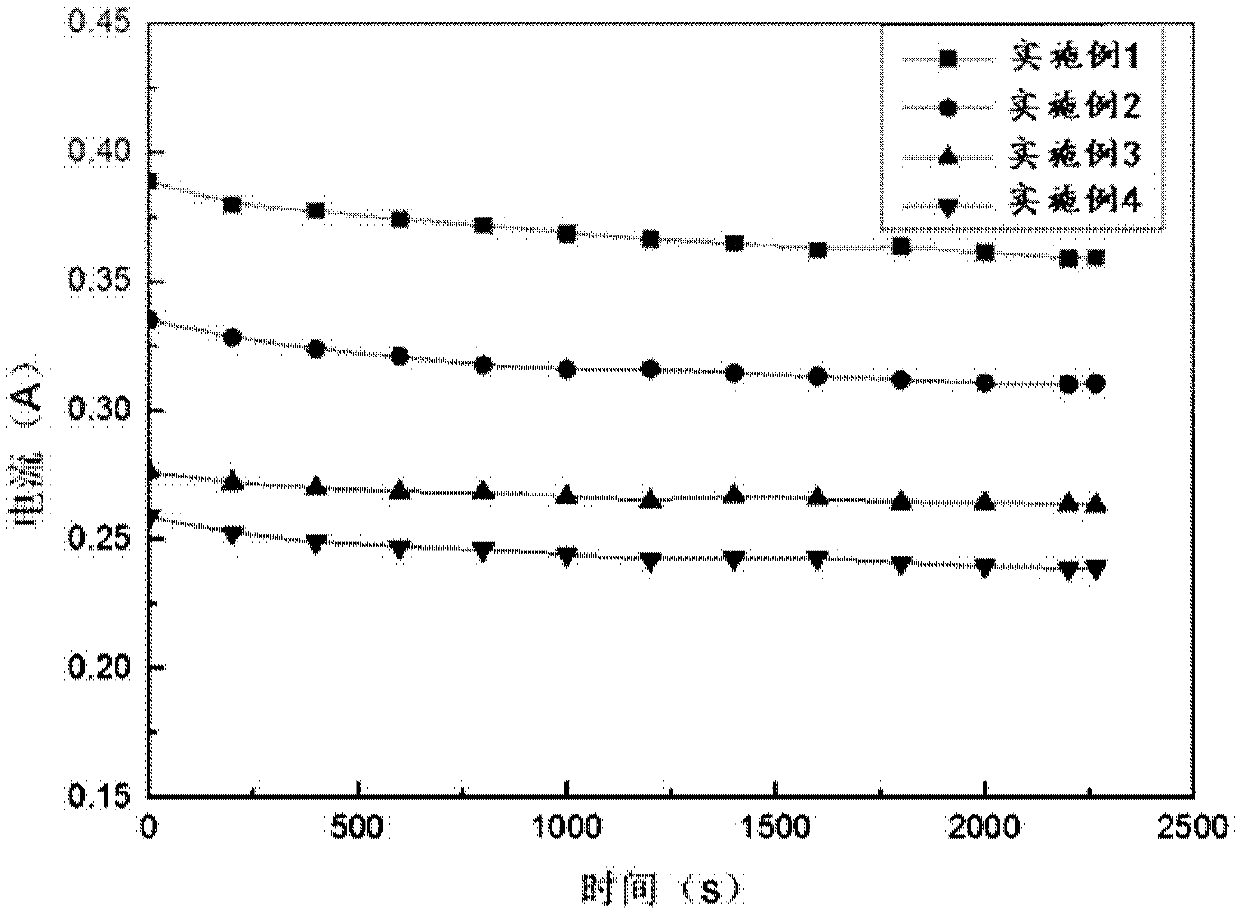
[0082] Measure the change of current and hydrogen production rate in the direct hydrogen production process of the salt difference, the results are shown in image 3 and Figure 4 ,in image 3 It is the current diagram in the direct hydrogen production process of the embodiments 1, 2, 3 and 4; Figure 4 It is the change diagram of hydrogen production rate in the process of direct hydrogen production by salt difference.
[0083] In this embodiment, the device can continuously generate current, and a stable hydrogen production rate of 1.89ml / min can be detected.
Embodiment 3
[0085] In a similar manner to Example 1 by figure 1 The equipment shown in that can directly produce hydrogen by using the salt difference, the difference is that the concentration of hydrochloric acid in the acid solution is changed to 0.5mol / L, and the concentration of sodium hydroxide in the lye is changed to Change to 0.5mol / L.
[0086] Measure the change of current and hydrogen production rate in the direct hydrogen production process of the salt difference, the results are shown in image 3 and Figure 4 ,in image 3 It is the current diagram in the direct hydrogen production process of the embodiments 1, 2, 3 and 4; Figure 4 It is the change diagram of hydrogen production rate in the process of direct hydrogen production by salt difference.
[0087] In this embodiment, the device can continuously generate current, and a stable hydrogen production rate of 1.79ml / min can be detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com