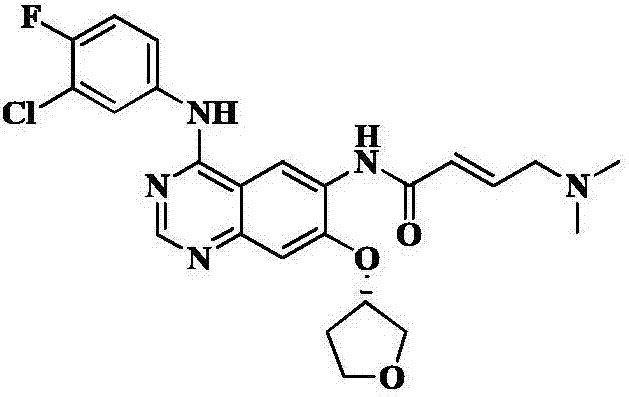
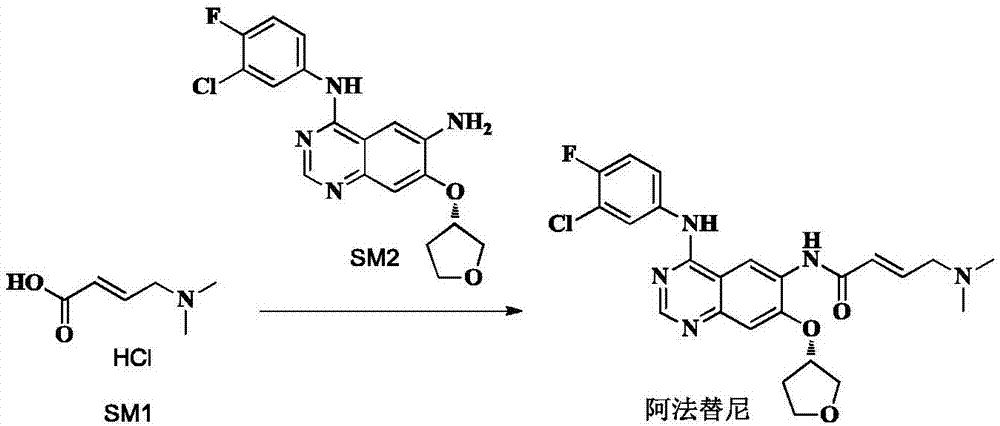
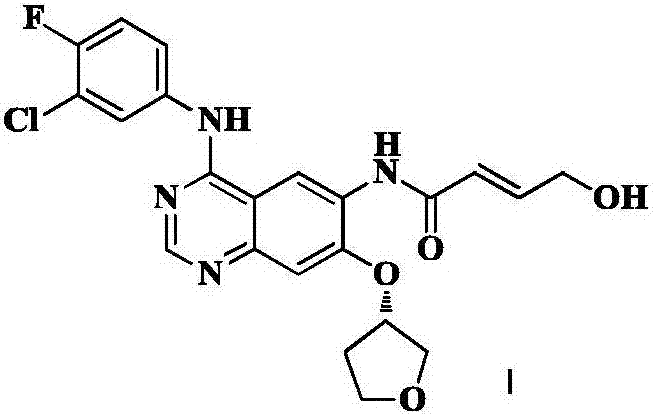
High-purity afatinib preparation method
A kind of afatinib, high-purity technology, applied in the field of drug synthesis, can solve the problems of unfriendly environment, unsuitable for industrial production, poor product purity, etc., and achieve the effect of simple and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Add 50g of trans-4-dimethylaminocroton hydrochloride, 125g of N-methylpyrrolidone, and 375g of ethyl acetate into a 1L three-necked flask, cool down to 0°C, and dropwise add chlorinated chlorinated 36.1 g of sulfone, after the dropwise addition, stirred at 0° C., monitored by HPLC until the reaction was complete (0.8% of SM1 remained).
[0059] (2) N4-(3-chloro-4-fluoro-phenyl)-7-(S)-tetrahydrofuran-3-yloxy)quinazoline-4,6,-diamine (SM2) 55g with N- 260 g of methylpyrrolidone was dissolved, and the solution was added dropwise to the reaction system of step (1). After the dropwise addition was completed, the reaction was carried out at 0° C., and the reaction was monitored by TLC until the SM2 reaction was complete.
[0060] (3) Control the temperature below 20°C, add 1.4kg of purified water dropwise to the system of step (2), adjust pH=9 with 20% by weight sodium hydroxide solution, extract with ethyl acetate 685g*2, and control the extraction temperature to Combin...
Embodiment 2
[0063] (1) Add 50g of trans-4-dimethylaminocroton hydrochloride, 125g of N-methylpyrrolidone, and 375g of ethyl acetate into a 1L three-necked flask, cool down to -5°C, and dropwise add chloride 36.1 g of sulfoxide, after the dropwise addition, stirred at -5°C, monitored by HPLC until the reaction was complete (SM1 residue 1.2%).
[0064] (2) N4-(3-chloro-4-fluoro-phenyl)-7-(S)-tetrahydrofuran-3-yloxy)quinazoline-4,6,-diamine (SM2) 45g with N- 225 g of methylpyrrolidone was dissolved, and the solution was added dropwise to the reaction system of step (1). After the dropwise addition was completed, the reaction was carried out at 0° C., and the reaction was monitored by TLC until the SM2 reaction was complete.
[0065] (3) Control the temperature below 20°C, add 1.35kg of purified water dropwise to the system in step (2), adjust the pH to 9 with 20% by weight sodium hydroxide solution, extract with ethyl acetate 675g*2, and control the extraction temperature to 45 °C, combine ...
Embodiment 3
[0068] (1) Add 50g of trans-4-dimethylaminocroton hydrochloride, 100g of N-methylpyrrolidone, and 400g of ethyl acetate into a 1L three-necked flask, cool down to 5°C, and dropwise add chlorinated chlorinated 36.1 g of sulfone, after the dropwise addition, stirred at 5°C, monitored by HPLC until the reaction was complete (1.8% of SM1 remained).
[0069] (2) N4-(3-chloro-4-fluoro-phenyl)-7-(S)-tetrahydrofuran-3-yloxy)quinazoline-4,6,-diamine (SM2) 45g with N- 225 g of methylpyrrolidone was dissolved, and the solution was added dropwise to the reaction system of step (1). After the dropwise addition was completed, the reaction was carried out at 0° C., and the reaction was monitored by TLC until the SM2 reaction was complete.
[0070] (3) Control the temperature below 20°C, add 1.35kg of purified water dropwise to the system in step (2), adjust the pH to 9 with 20% by weight sodium hydroxide solution, extract with ethyl acetate 675g*2, and control the extraction temperature to 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com