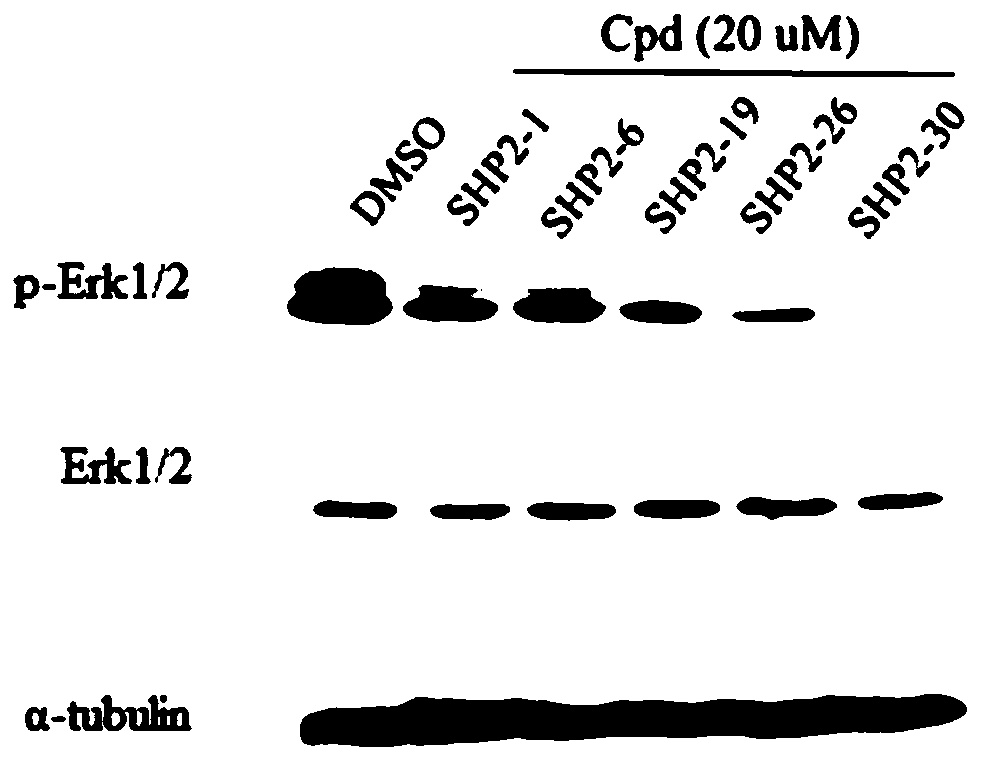
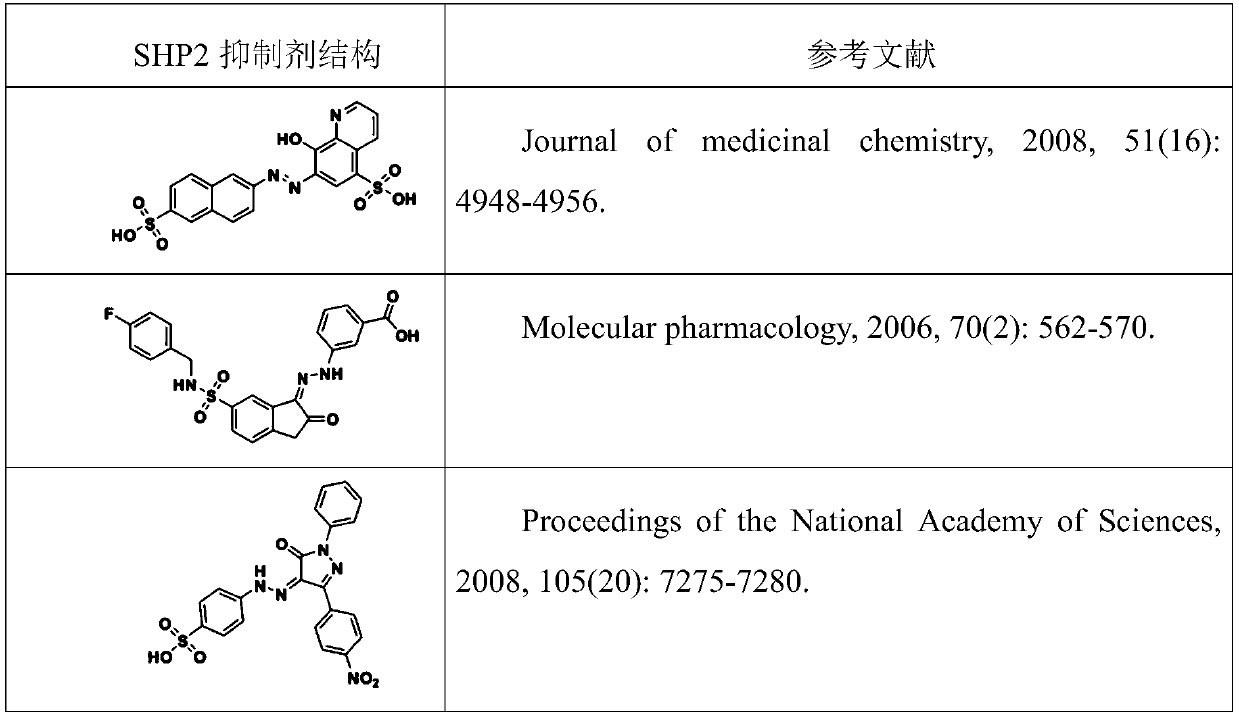
N-heterocyclic compounds, their intermediates, preparation methods, pharmaceutical compositions and applications
A compound and heterocyclic technology, applied in the field of N-heterocyclic compounds, can solve problems such as poor selectivity and poor cell permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] The synthesis of embodiment 1SHP2-28
[0124] Synthesis of S1
[0125]
[0126] Add 6-bromo-2-naphthoic acid (251.1mg, 1.0mmol) into a 25mL dry egg-shaped bottle, replace the nitrogen three times to make the system in a nitrogen atmosphere, add 5mL of freshly distilled dichloromethane to dissolve it, and then slowly Thionyl chloride (0.36mL, 5.0mmol) was added dropwise, and after the dropwise addition, a catalytic amount of ultra-dry N,N-dimethylamide was added to promote the reaction. After heating to reflux for 30 minutes, stop the heating, spin the solvent dry, pump it with an oil pump for half an hour, and directly put it into the next reaction. In the second step, N, O-dimethylhydroxylamine hydrochloride (107.3mg, 1.1mmol) and triethylamine (0.42mL, 3.0mmol) were added to a 25mL dry egg-shaped bottle, and 5mL of dichloromethane was added to make the raw material completely Dissolve, cool the system to 0°C in an ice-water bath, slowly add the acid chloride newl...
Embodiment 2
[0153] The synthesis of embodiment 2 compound SHP2-30
[0154] Synthesis of S7
[0155]
[0156] Add 6-bromo-2-naphthoic acid (2.4996g, 10.0mmol) into a 50mL dry egg-shaped flask, add 20mL of anhydrous methanol to dissolve it, then slowly add 1mL of concentrated sulfuric acid dropwise, and reflux the system overnight. TLC tracked until the conversion of the raw materials was completed, the heating was stopped, and after cooling to room temperature, saturated aqueous sodium carbonate solution was added to quench the reaction. The reaction system was adjusted to be neutral, extracted with ethyl acetate, and the organic phase was washed three times with water and dried with anhydrous sodium sulfate. Concentration under reduced pressure gave white solid S7 (2.63 g, yield 100%).
[0157] 1 H NMR (300MHz, CDCl 3 )δ8.57(s,1H),8.11–8.03(m,2H),7.81(t,J=8.3Hz,2H),7.62(dd,J=8.4,1.5Hz,1H),3.98(s,3H ).
[0158] Synthesis of S8
[0159]
[0160] Compound S7 (265.1mg, 1.0mmol), ...
Embodiment 3
[0175] The synthesis of embodiment 3 compound SHP2-31
[0176] Synthesis of S11
[0177]
[0178] 2-Chloronaphthalene (325.2mg, 2.0mmol) and aluminum trichloride (320mg, 2.4mmol) were dissolved in 4mL of carbon disulfide solution, the system was cooled to 0°C in an ice-water bath, and acetyl chloride (0.16mL, 2.2 mmol), after the dropwise addition, it was incubated at 0° C. for 10 minutes, and then returned to room temperature and stirred overnight. TLC tracked until the conversion of the raw materials was completed, the reaction solution was poured into ice water, and concentrated hydrochloric acid was added dropwise to adjust the pH of the reaction solution to 2.0. Then it was extracted with dichloromethane, and the organic phase was washed with saturated aqueous sodium bicarbonate until neutral. The organic phase was washed several times with water and dried over anhydrous sodium sulfate. Concentration under reduced pressure to obtain the crude product was separated a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com