Preparation and application of sulfo-carbamate hypochlorous acid fluorescent probe
A technology of hypochlorous acid and preparation, which is applied in the directions of fluorescence/phosphorescence, luminescent materials, material analysis by optical means, etc., can solve the problems of poor selectivity, complex synthesis, low sensitivity, etc., and achieves good stability, simple synthesis and low cost. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] (Scheme 1) Tricyanotrimethylfuran (398 mg, 2 mmol) and phenyl p-formylthiocarbamate (419 mg, 2 mmol) were dissolved in 20 mL of absolute ethanol, and then diisopropylethyl Amine (646mg, 3mmol), the reaction solution was reacted at room temperature for 6 hours. After the reaction was completed, the solvent was removed by rotary evaporation to obtain a crude product, which was then separated and purified with a silica gel column (dichloromethane: petroleum ether = 2:1 as the eluent) to obtain 648 mg of a yellow solid with a yield of 83%.
[0035] (Scheme 2) Tricyanotrimethylfuran (398 mg, 2 mmol) and phenyl p-formylthiocarbamate (460 mg, 2.2 mmol) were dissolved in 20 mL of absolute ethanol, and then diisopropylethyl amine (646mg, 3mmol), and the reaction solution was reacted at room temperature for 6 hours. After the reaction was completed, the solvent was removed by rotary evaporation to obtain a crude product, which was then separated and purified with a s...
Embodiment 2
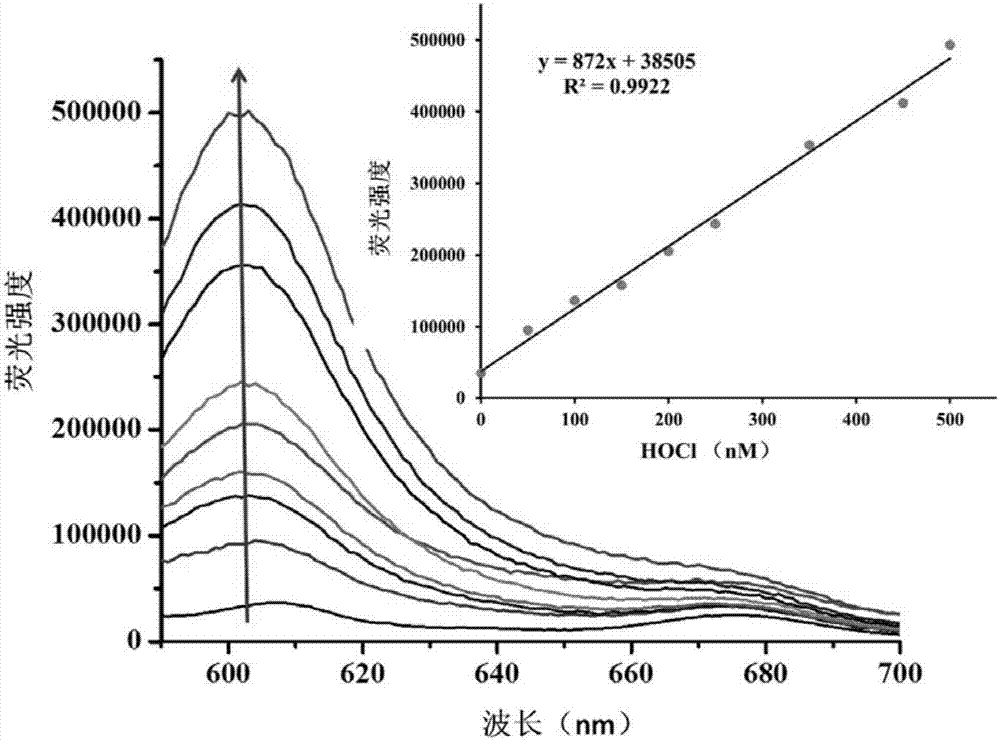
[0039] Under the condition of PBS pH=7.4, the impact of different concentrations of hypochlorous acid on the fluorescence spectrum of the probe solution, the illustration is the linear relationship diagram of the quantitative analysis of hypochlorous acid by fluorescence spectroscopy; the above-mentioned determination is carried out in the aqueous solution of 10mM PBS, so The probe used was the probe prepared in Example 1, and all spectral tests were measured at 25° C. after the addition of hypochlorous acid for 1 min. See results figure 1 .
[0040] figure 1 is the graph of the change of the fluorescence spectrum of the probe with different concentrations of hypochlorous acid. It can be seen from the figure that the probe has almost no fluorescence before adding hypochlorous acid; after adding hypochlorous acid, the fluorescence intensity at 603nm gradually increases with the increase of hypochlorous acid concentration. Moreover, a good linear relationship is maintained be...
Embodiment 3
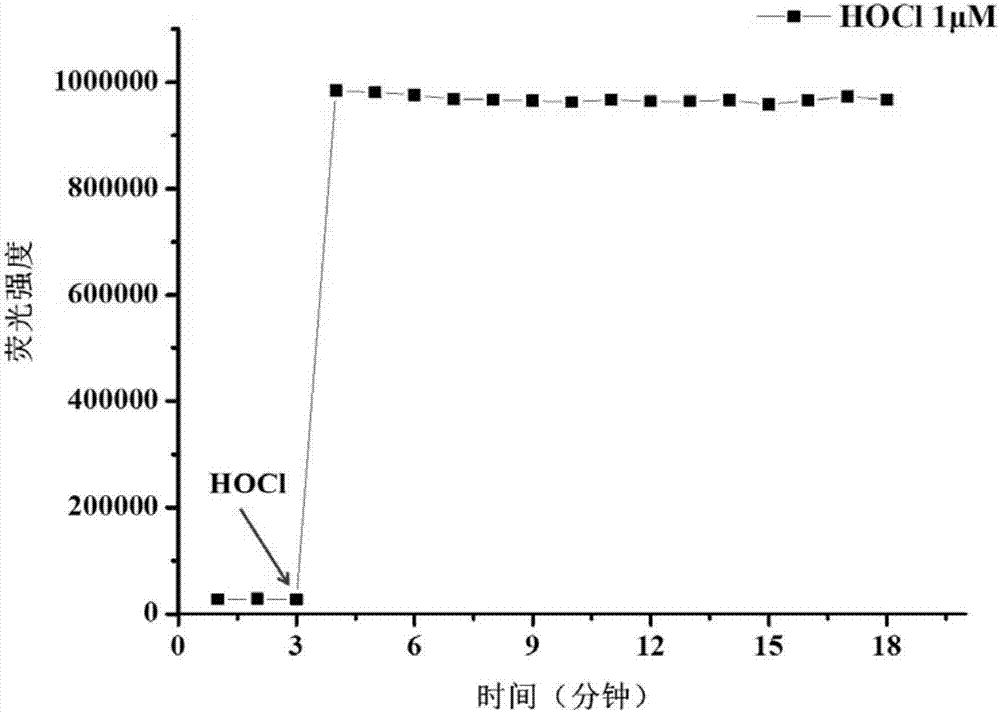
[0042]Test results of probe (5 μM) response time to hypochlorous acid (5 μM). The above determinations were carried out in 10mM PBS, pH 7.4 aqueous solution, the probe used was the probe prepared in Example 1, and all spectral tests were measured at 25°C. See results figure 2 .
[0043] from figure 2 It can be seen that after adding an equal concentration of hypochlorous acid, the fluorescence intensity rises suddenly, and the response can be completed within 1 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com