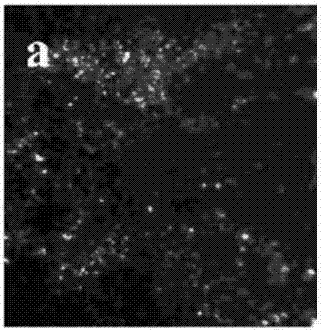
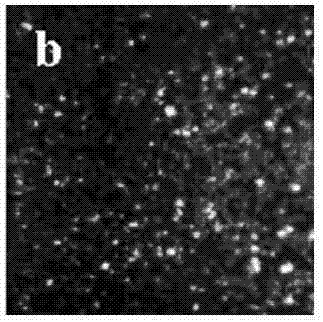
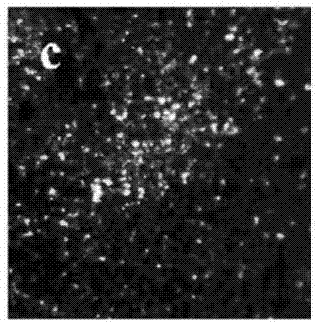
Electrolyte capable of suppressing zinc dendritic crystal growth in charging-discharging process of Zn-PANI secondary battery
A charging and discharging process, secondary battery technology, applied in secondary batteries, acidic electrolytes, aqueous electrolytes, etc., can solve problems such as short cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] One, the preparation of zinc sheet:
[0024] Zinc flakes are 99.99% pure and cut to 1×10 cm 2 The long strips are polished with metallographic sandpaper to remove surface oxides, then washed with secondary water and dried.
[0025] Second, the preparation of battery electrolyte:
[0026] A certain amount of ZnCl 2 and NH 4 Dissolve Cl in twice distilled water, then add one or more of dicarboxylic acid, dicarboxylic acid salt, tricarboxylic acid salt and one or more of surfactant, and then add gas phase SiO 2 , after adjusting the pH value of the mixed solution to 3.0-6.0 with hydrochloric acid or sodium hydroxide, fully stir for 5-12 h.
[0027] The following table is the composition range of the prepared electrolyte:
[0028]
[0029] The above dicarboxylic acid may be at least any one of oxalic acid, malonic acid, succinic acid, adipic acid, maleic acid, phthalic acid or phthalic acid.
[0030] Dibasic carboxylates can be sodium oxalate, sodium malonate, sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com