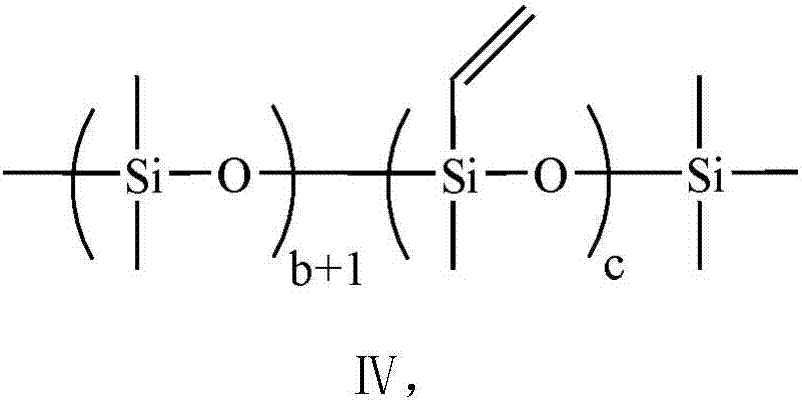
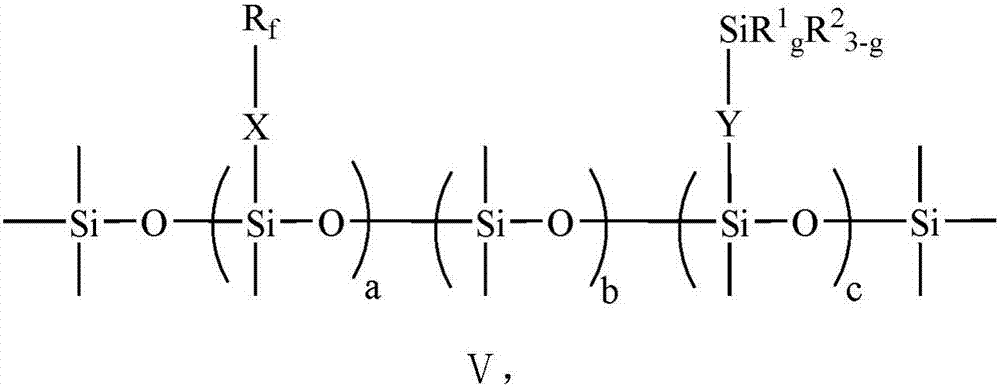
Preparation method of perfluoropolyfluoroether fluorosilane with plurality of hydrolytically-active end groups
A perfluoropolyether-based, fluorosilane-based technology, used in biocide-containing paints, antifouling/underwater coatings, coatings, etc., can solve the problems of inflammability and explosion, difficult to obtain raw materials, low surface tension and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] This embodiment provides a method for preparing perfluoropolyfluoroether-based fluorosilane with multiple hydrolysis active end groups, comprising the following steps:
[0076] (1) In a four-neck flask equipped with a reflux condenser, nitrogen purging, strong stirring and temperature control device, add 100.0g vinyl-terminated perfluoropolyether (molecular weight: 1684g / mol, Taicang Sinochem Environmental Protection Chemical Co., Ltd. Provided, the general structural formula is: F(CFCF 3 CF 2 O) 9 CFCF 3 CH 2 OCH 2 CH=CH 2 ), 6.3g methyldimethoxyhydrogensilane and 110.0g m-ditrifluorotoluene, stirred evenly at room temperature, and added 1g Karstedt type platinum catalyst VM-23 (Pt content is 3000ppm, Zhejiang Quzhou Jiancheng Organic Silicon Material Co., Ltd. Provided by the company), gradually raise the temperature to 80°C, then keep the temperature for 8 hours, and finally remove the low boiling point for 2 hours under a vacuum of 0.2KPa to obtain the product...
Embodiment 2
[0089] This example provides a method for preparing a perfluoropolyfluoroether-based fluorosilane with multiple hydrolysis active end groups, referring to the method and steps in Example 1, the difference is that the vinyl-terminated perfluoropolyether in the formula The specification is changed to: the molecular weight is 3510g / mol, provided by Taicang Sinochem Environmental Protection Chemical Co., Ltd., and the general structural formula is: F(CFCF 3 CF 2 O) 20 CFCF 3 CH 2 OCH 2 CH=CH 2 , and the corresponding mass is replaced with 209g.
[0090] The perfluoropolyfluoroether fluorosilane prepared in this example is denoted as PFPE-FS-2, and its structural formula conforms to that shown in formula V, in formula V: a=1, b=4, c=4, d=3, e=20, g=3, X=CFCF 3 CH 2 OCH 2 CH 2 CH 2 , Y=CH 2 CH 2 CH 2 , R 1 =OC 2 h 5 .
Embodiment 3
[0092] This example provides a method for preparing a perfluoropolyfluoroether-based fluorosilane with multiple hydrolysis active end groups, referring to the method and steps in Example 1, the difference is that the vinyl-terminated perfluoropolyether in the formula The specification is changed to: the molecular weight is 5170g / mol, provided by Taicang Sinochem Environmental Protection Chemical Co., Ltd., and the general structural formula is: F(CFCF 3 CF 2 O) 30 CFCF 3 CH 2 OCH 2 CH=CH 2 , and the corresponding mass is replaced with 307g.
[0093] The perfluoropolyfluoroether fluorosilane prepared in this example is denoted as PFPE-FS-3, and its structural formula conforms to that shown in formula V, in formula V: a=1, b=4, c=4, d=3, e=30, g=3, X=CFCF 3 CH 2 OCH 2 CH 2 CH 2 , Y=CH 2 CH 2 CH 2 , R 1 =OC 2 h 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com