Silver-coated copper powder and method for manufacturing same
A technology of silver-coated copper powder and manufacturing method, which is applied in the directions of coating, conductor, transportation and packaging, etc., can solve the problems of storage stability (poor reliability, high cost, etc.), and achieve the effect of excellent reliability and improved conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
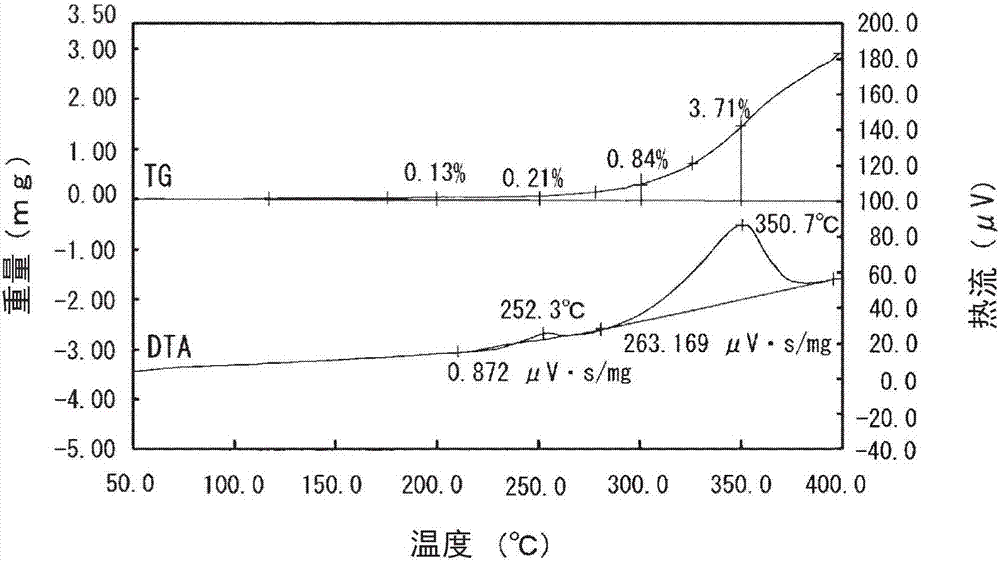
[0048] Prepare a commercially available copper powder manufactured by the atomization method (atomized copper powder SF-Cu 5μm manufactured by Nippon Atomization Processing Co., Ltd. (Nippon Atmos Processing Co., Ltd.), and calculate the copper powder (before silver coating) Particle size distribution, the cumulative 10% particle size of copper powder (D 10 ) Is 2.26μm, 50% cumulative particle size (D 50 ) Is 5.20μm, 90% cumulative particle size (D 90 ) Is 9.32μm. In addition, the particle size distribution of the copper powder was measured with a laser diffraction particle size distribution device (MICROTRAC particle size distribution measuring device MT-3300 manufactured by Nikkiso Co., Ltd.), and the cumulative 10% particle size (D 10 ), cumulative 50% particle size (D 50 ), cumulative 90% particle size (D 90 ).
[0049] In addition, the following solution was prepared: a solution obtained by dissolving 1470g EDTA-4Na (43%) and 1820g ammonium carbonate in 2882g pure water (solu...
Embodiment 2
[0055] As the silver supporting liquid, in addition to using 1.67g of 100g / L silver potassium cyanide (acid concentration 60g / L) mixed with 0.1g tripotassium citrate monohydrate, 0.082g citric anhydride, 0.017gL-aspartic Except for the aqueous solution obtained by the acid and 2 g of water, by the same method as in Example 1, a silver-coated copper powder with silver supported on the surface was obtained. In addition, the concentrations of Ag and Cu in the filtrate were measured with an ICP mass analyzer (ICP-MS), and the results were 2 mg / L and 180 mg / L, respectively.
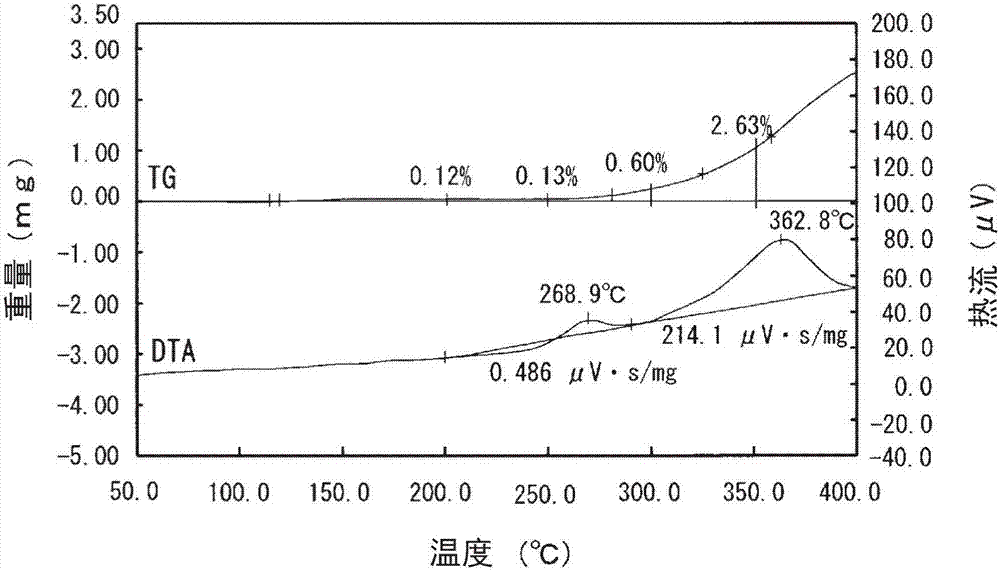
[0056] The Ag content in the thus-obtained silver-coated copper powder (supporting silver on the surface) was calculated by the same method as in Example 1. As a result, it was 10.84% by mass. In addition, the amount of silver supported on the surface was calculated by the same method as in Example 1. As a result, it was 0.64% by mass.
[0057] In addition, the weight increase rate of the silver-coated copper po...
Embodiment 3
[0059] As the silver supporting liquid, except that 0.2 mL of the silver supporting liquid taken from 1 g of an aqueous solution containing 100 g / L of silver potassium cyanide was used, the same method as in Example 1 was used to obtain a silver supporting liquid on the surface. Coated with silver copper powder. In addition, the concentrations of Ag and Cu in the filtrate were measured with an ICP mass spectrometer (ICP-MS), and the results were less than 1 mg / L and 44 mg / L, respectively.
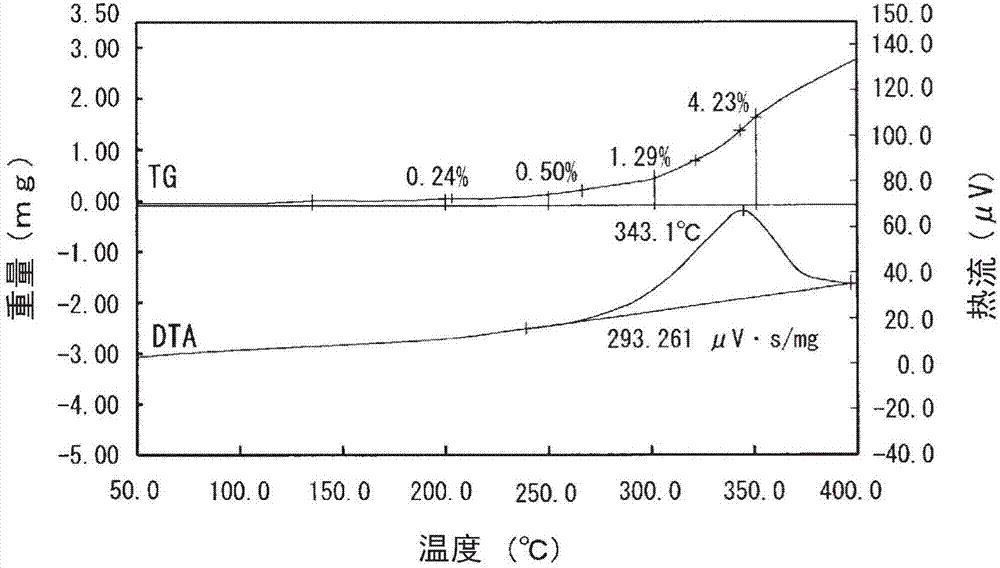
[0060] The Ag content in the thus-obtained silver-coated copper powder (supporting silver on the surface) was calculated by the same method as in Example 1. As a result, it was 10.50% by mass. In addition, the amount of silver supported on the surface was calculated by the same method as in Example 1. As a result, it was 0.30% by mass.
[0061] In addition, the weight increase rate of the silver-coated copper powder (supporting silver on the surface) at 200°C, 250°C, 300°C, and 350°C was calcul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com