Method for extracting lithium from lithium-contained solution based on LiMn2O4 electrode material
A technology of electrode material and lithium solution, which is applied in the electrochemical field of extracting lithium from lithium-containing solution based on lithium battery electrode materials, can solve problems such as low lithium insertion efficiency, corrosion of equipment environment, hazards, etc., and achieves convenient operation and environmental friendliness , The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Preparation of lithium-rich electrodes:
[0035] LiMn 2 o 4 After the powder is fully mixed with the conductive agent acetylene black and the binder PTFE according to the mass ratio of 80:10:10, add an appropriate amount of ethanol to make it into a slurry, and apply it on the surface of the carbon cloth. The effective area of the carbon cloth is 3.5×3cm 2 , the coating quality is about 10mg / cm 2 , placed at 70°C for 12h and dried to make lithium-rich LiMn 2 o 4 electrode.
[0036] (2) Preparation of lithium-poor electrode:
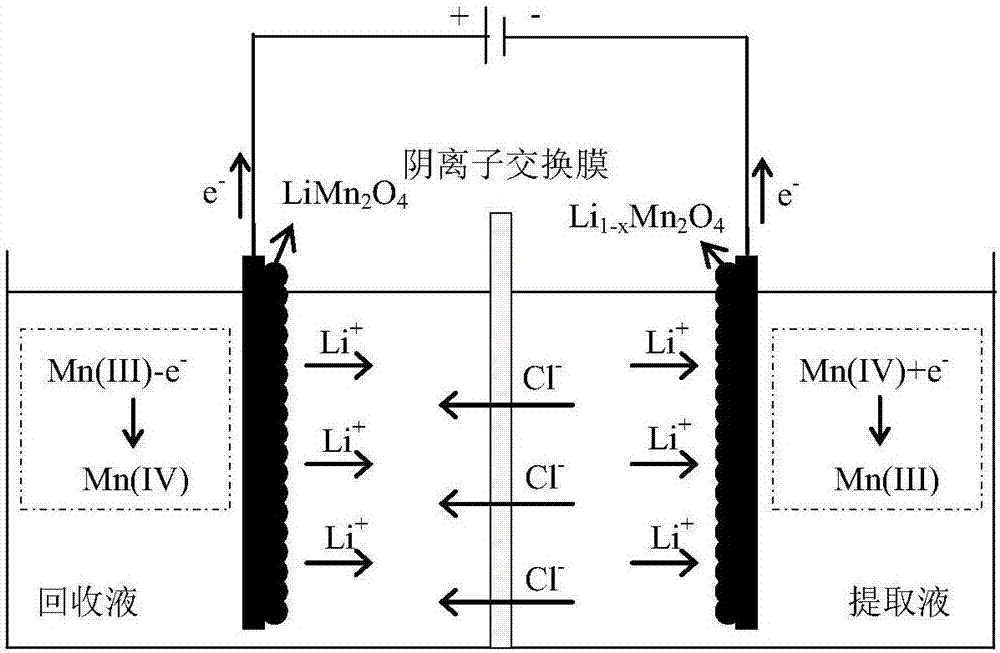
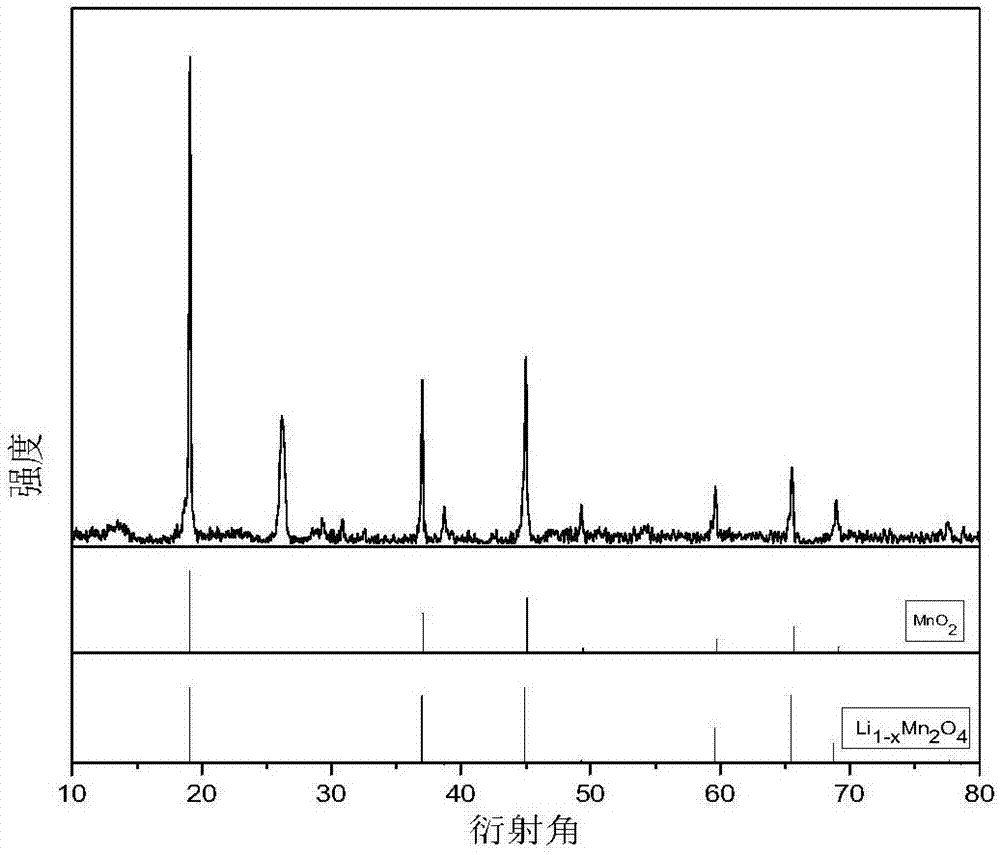
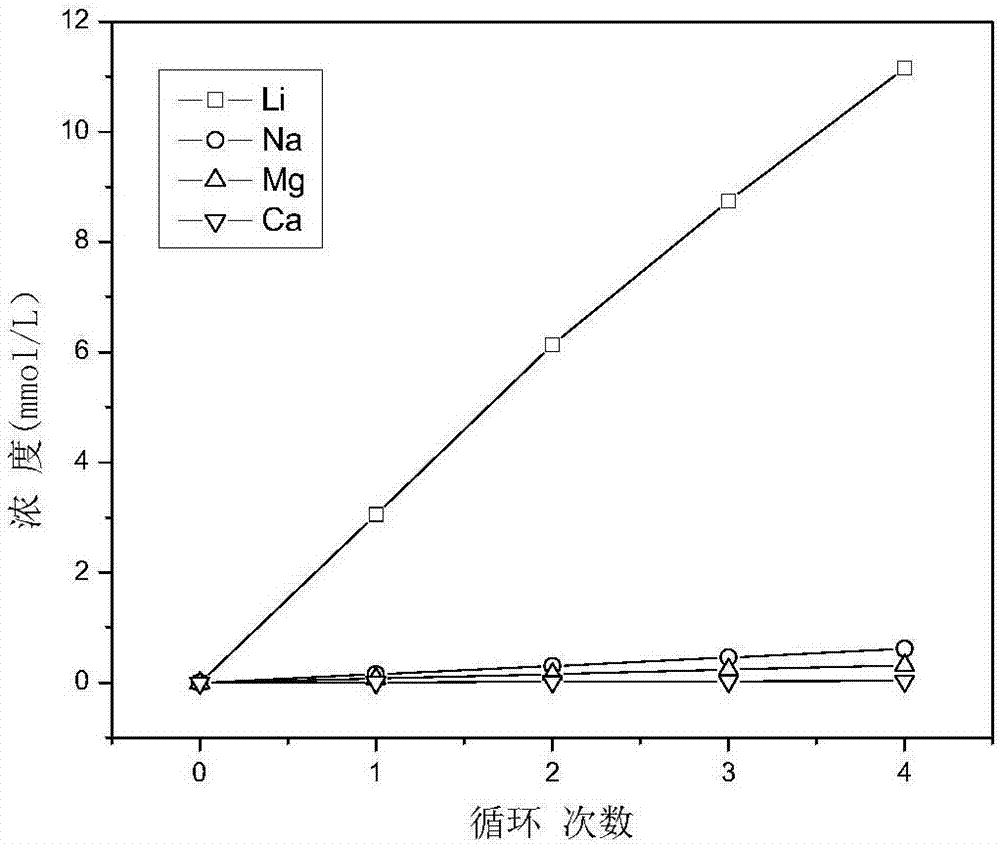
[0037] Take LiMn 2 o 4 The electrode is the positive electrode (i.e., the anode), and the AgCl electrode is used as the negative electrode (i.e., the cathode), which is placed in an electrolytic cell, and 0.05mol / L KCl solution is used as the electrolyte, and electrochemical delithiation is carried out at a constant voltage of 0.8V. When the current value is as low as 0.2mA, the reaction stops, and lithium-poor Li 1-x mn 2 o 4 elec...
Embodiment 2
[0046] (1) Preparation of lithium-rich electrodes:
[0047] LiMn 2 o 4 After the powder is fully mixed with the conductive agent acetylene black and the binder PTFE according to the mass ratio of 80:10:10, add an appropriate amount of ethanol to make it into a slurry, and apply it on the surface of the carbon cloth. The effective area of the carbon cloth is 3.5×3cm 2 , the coating quality is about 8mg / cm 2 , placed at 70°C for 12h and dried to make lithium-rich LiMn 2 o 4 electrode.
[0048] (2) Preparation of lithium-poor electrode:
[0049] Take LiMn 2 o 4 The electrode is the positive electrode (i.e., the anode), and the AgCl electrode is used as the negative electrode (i.e., the cathode), which is placed in the electrolytic cell, and 0.05mol / L KCl solution is used as the electrolyte, and the electrochemical oxidation is carried out at a voltage of 0.8V. When the current value is as low as 0.2mA, the reaction stops, and lithium-poor Li 1-x mn 2 o 4 electrode. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com