Hydrogenation method of butyronitrile latex
A technology for nitrile rubber and hydrogenation reaction is applied in the field of hydrogenation of nitrile latex, which can solve the problems that the hydrogenation degree of the hydrogenation product needs to be improved, the reaction activity of the hydrogenation catalyst is low, and the gel content of the product is high, and the reaction is shortened. Time, short response time, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
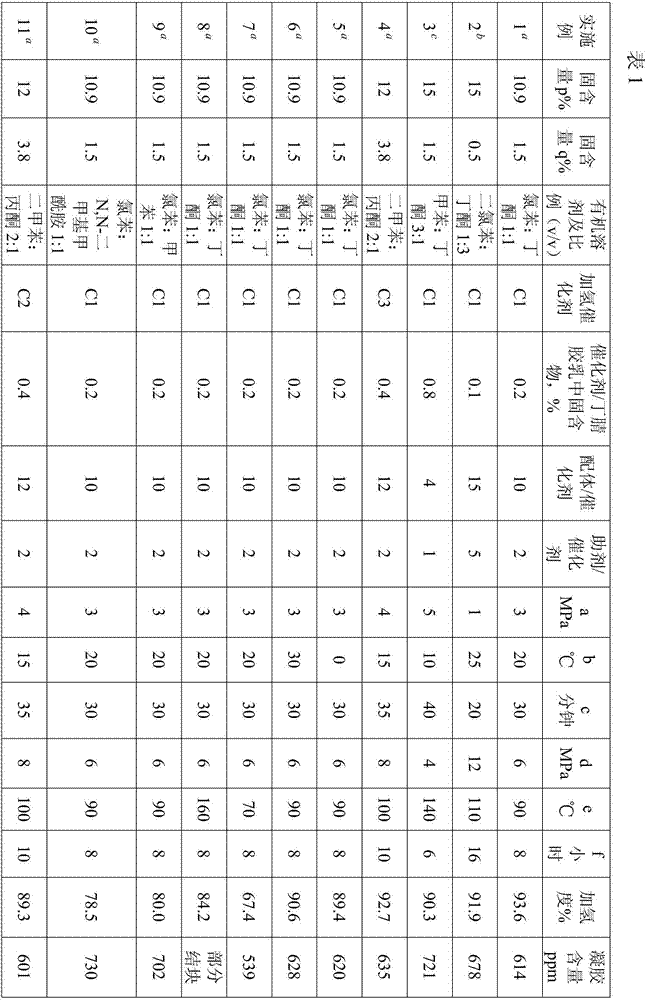
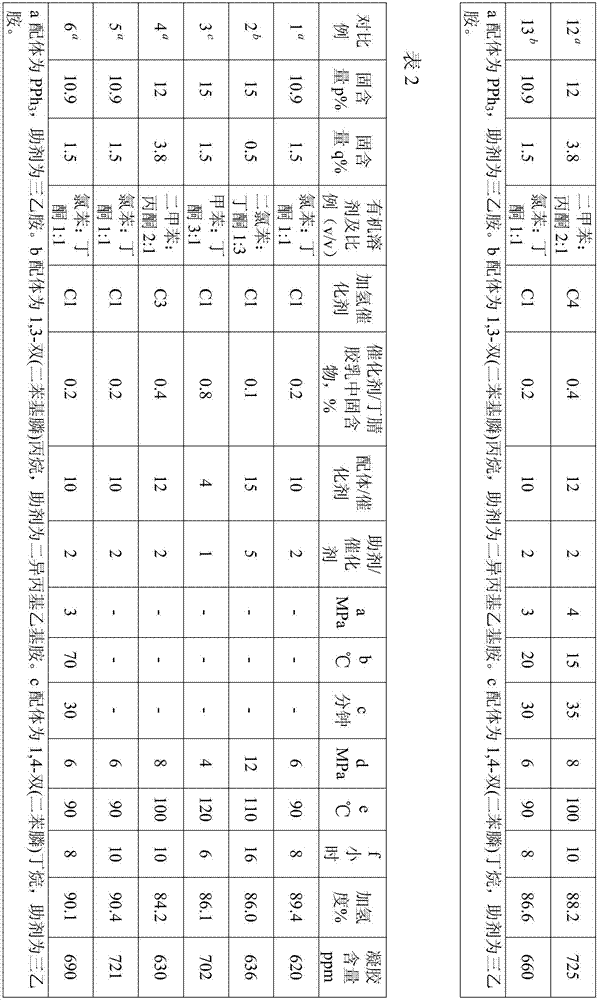
Examples
Embodiment
[0040] The following examples will further illustrate the present invention, but do not limit the present invention thereby.
[0041] In the following examples and comparative examples, the method for determining the degree of hydrogenation is to test according to the method in the literature (nuclear magnetic analysis method for calculating the microstructure of hydrogenated nitrile rubber and the degree of hydrogenation. Petrochemical Industry, 2014, 43, 403).
[0042] Determination method of gel content: Weigh w1 gram of dry glue, add chlorobenzene to make a solution of 1g / 100mL, after dissolving for 24 hours, pour the solution into a filter screen with constant weight (w2 grams) to filter, and then put the The filter screen with insoluble matter is dried to constant weight, and the weight is recorded as w3 grams, then the gel content is: (w3-w2) / w1*10 6 ppm.
[0043] Hydrogenation catalyst (PPh 3 ) 3 RhCl was purchased from Acros Company and was denoted as C1.
[0044]...
preparation example 1
[0047] This preparation example is used to illustrate hydrogenation catalyst M 1 a m 2 b x m L n The preparation method of (C2), wherein, M 1 for rhodium, M 2 is ruthenium, X is chlorine, L is triphenylphosphine, a:b:m:n is 2:1:4:9.
[0048] 4.5 g PPh 3 Dissolve in hot ethanol solution at 70°C under the protection of argon, and add the dissolved solution into the reaction flask; weigh 0.5 g of RhCl 3 .H 2 O and 0.23 g RuCl 3 .H 2 O was dissolved in ethanol solution under the protection of argon, heated to reflux at 78°C, and RhCl 3 .H 2 O and RuCl 3 .H 2 The ethanol solution of O was added dropwise into the reaction flask to react for 2.5 hours, cooled to 25°C, filtered with suction, washed with ether to obtain a powdered hydrogenation catalyst with Rh:Ru=2:1, denoted as C2.
preparation example 2
[0050] This preparation example is used to illustrate hydrogenation catalyst M 1 a m 2 b x m L n The preparation method of (C3), wherein, M 1 for rhodium, M 2 is ruthenium, X is chlorine, L is triphenylphosphine, a:b:m:n is 3:1:5:12.
[0051] 6 g PPh 3 Dissolve in hot ethanol solution at 70°C under the protection of argon, and add the dissolved solution into the reaction flask; weigh 0.75 g of RhCl 3 .H 2 O and 0.23 g RuCl 3 .H 2 O was dissolved in ethanol solution under the protection of argon, heated to reflux at 78°C, and RhCl 3 .H 2 O and RuCl 3 .H 2 The ethanol solution of O was added dropwise into the reaction flask to react for 2.5 hours, cooled to 25°C, filtered with suction, and washed with ether to obtain a powdered hydrogenation catalyst with Rh:Ru=3:1, denoted as C3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com