A kind of metformin hydrochloride sustained-release tablet and preparation method thereof
A technology of metformin hydrochloride and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., to achieve the effects of small tablet weight differences, excellent water retention, and avoiding the use of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0037] The preparation of embodiment 1-5 and comparative example 1-7 metformin hydrochloride sustained-release tablet
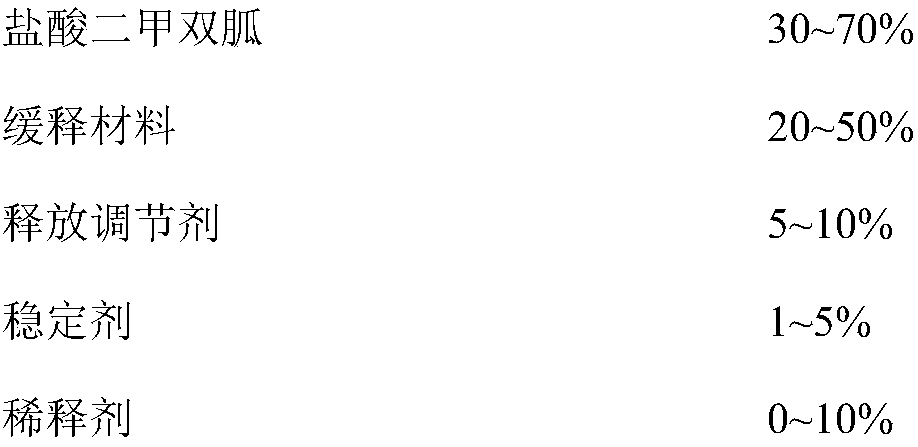
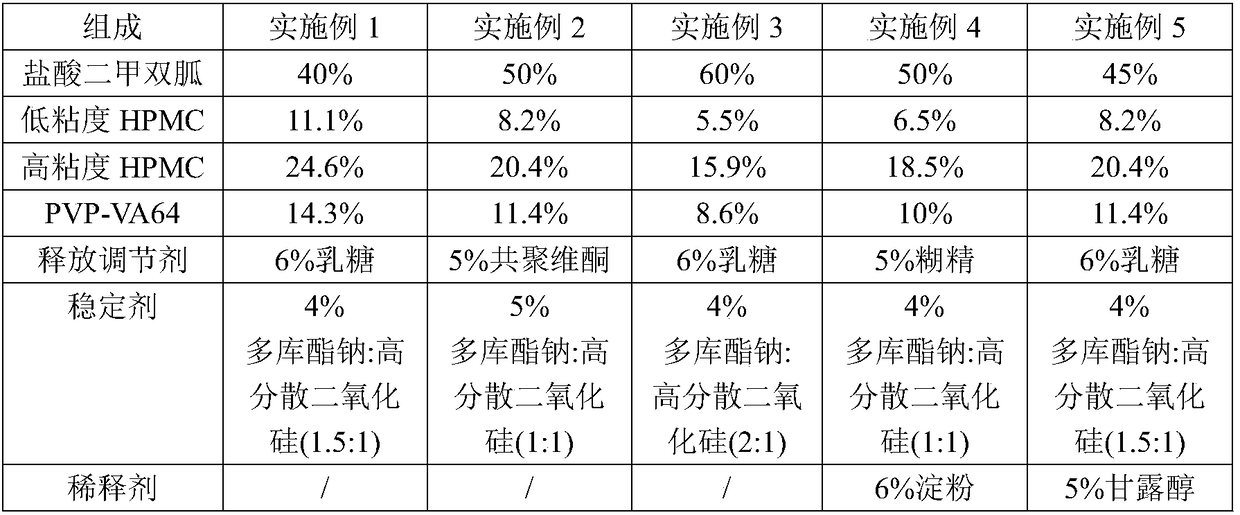
[0038] The composition of embodiment 1-5 and comparative example 1-7 metformin hydrochloride sustained-release tablet is shown in the table below:
[0039] Among them, the viscosity of low-viscosity HPMC is 500-1000 mPa.s; the viscosity of high-viscosity HPMC is 10000-15000 mPa.s.
[0040]
[0041] The formula of comparative example 1-7 metformin hydrochloride sustained-release tablet is basically the same as that of Example 1, and the differences are shown in the table below:
[0042]
[0043]
Embodiment 1
[0044] The preparation of embodiment 1 metformin hydrochloride slow-release tablet comprises the following steps:
[0045] (1) respectively pulverize metformin hydrochloride, low-viscosity HPMC, high-viscosity HPMC, PVP-VA64, lactose, docusate sodium, and high-dispersion silicon dioxide, pass through a 100-mesh sieve, and mix uniformly in proportion to make a physical mixture;
[0046] (2) Set the extrusion temperature of the twin-screw extruder to 150°C, start the screw after the temperature rises to the set temperature, set the screw speed to 80 rpm, and add the physical mixture in step (1) to the extrusion In the machine, it is melted, extruded, and finally extruded in strips;
[0047] (3) After the strip-shaped extrudate is cooled, it is pulverized and passed through a 60-mesh sieve to obtain a drug solid or powder;
[0048] (4) The granules obtained in step (3) are directly compressed into tablets.
[0049] Examples 2-5 and Comparative Examples 1-7 Refer to Example 1 fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com