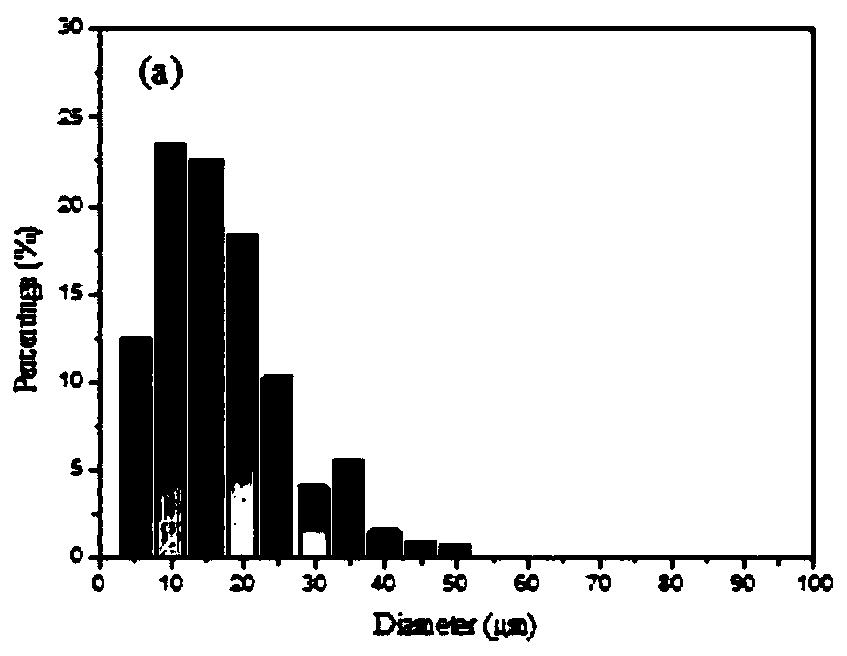
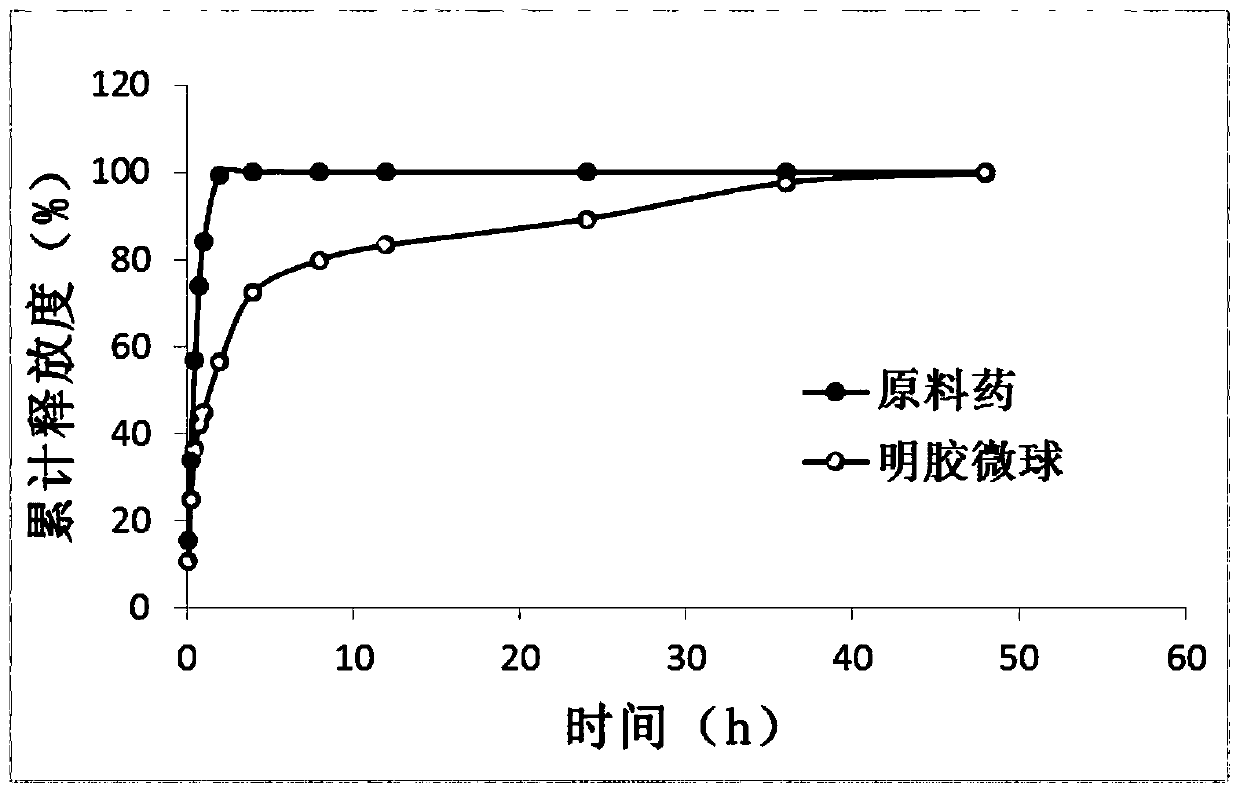
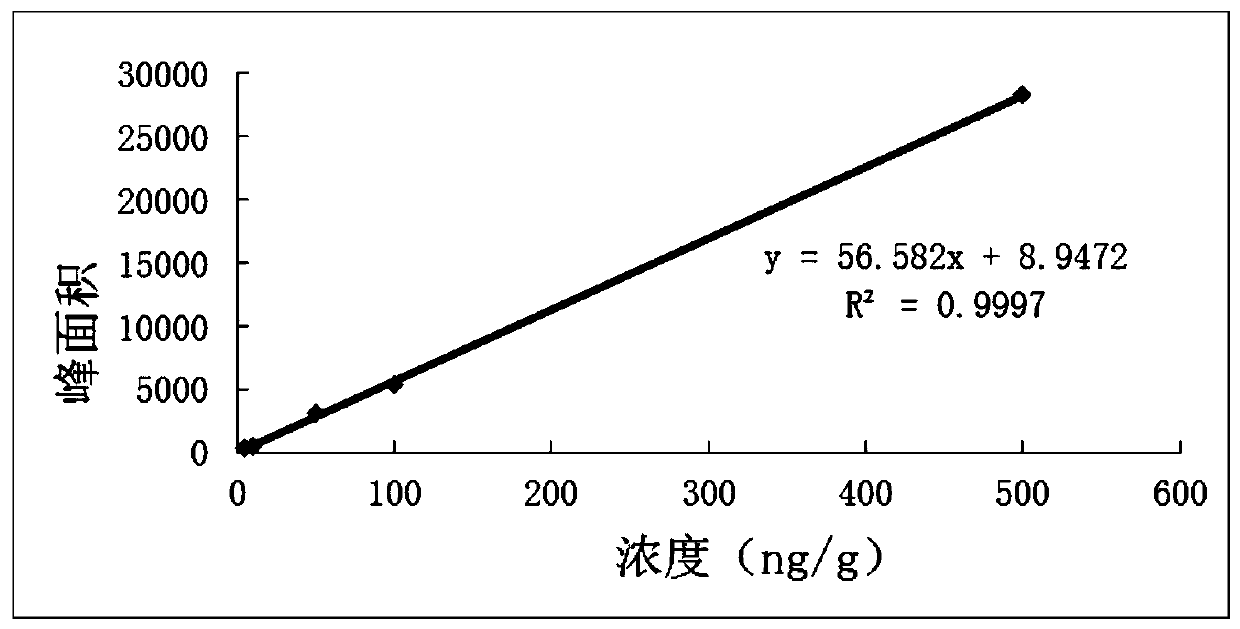
A kind of lung targeting cefquinol sulfate gelatin microspheres and preparation method thereof
A quinol gelatin and lung-targeting technology, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, and medical preparations containing active ingredients. No problem, inconvenient clinical application, etc., to achieve the effect of high reproducibility, easy control of microsphere particle size, and excellent precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention is achieved through the following measures: a lung targeting cefquinol sulfate gelatin microspheres, the preparation method comprising the following steps:
[0028] In the first step, the raw material drug and the dispersant are mixed according to the ratio of 1g: 2-5ml, put into the frequency conversion planetary ball mill, ball mill for 1-3h, intermittently for 0.5-1.5h, and then ball mill for 1-3h, evaporate the liquid, and get the treatment Subsequent raw materials;
[0029] In the second step, weigh gelatin and sodium alginate as carriers, dissolve them in double distilled water at 40-50°C, heat and stir until they are completely dissolved, then add cefquinol raw materials in proportion, and mix them evenly by ultrasonic waves. 300-400w ultrasonic for 1-2min, high power 600-1000w ultrasonic for 1-2min, add flow aid, mix well to form a mixture, then add glutaraldehyde solution, and then use double-flow spray dryer for spray drying, and magnetic...
Embodiment 1
[0158] The preparation method comprises the following steps:
[0159] In the first step, mix 3.3g of raw material medicine with 16ml of dispersant, put it into a frequency conversion planetary ball mill, ball mill for 2 hours, intermittently for 1 hour, ball mill for another 2 hours, and evaporate the liquid to obtain the treated raw medicine;
[0160] In the second step, weigh 10g of gelatin and 3.3g of sodium alginate as carriers, dissolve them in 250ml of double-distilled water at 50°C, heat and stir until completely dissolved, then add the cefquinol raw material, and mix them evenly by ultrasonic waves. 400w ultrasonic for 1min, high power 1000w ultrasonic for 1min, add 0.133g flow aid, mix to form a mixture, then add glutaraldehyde solution, then spray dry with double-flow spray dryer, and need magnetic stirring at the same time, spray dry to get microspheres.
[0161] Wherein, in the dispersant, the volume ratio of ethanol:isopropanol:dichloromethane is 5:2:1.
[0162] ...
Embodiment 2
[0169] The preparation method comprises the following steps:
[0170] In the first step, mix 1.1g of raw material medicine with 5ml of dispersant, put it into a frequency conversion planetary ball mill, ball mill for 1 hour, intermittently for 0.5 hour, ball mill for another 1 hour, and evaporate the liquid to obtain the treated raw material medicine;
[0171] In the second step, weigh 10g of gelatin and 1g of sodium alginate as the carrier, dissolve them in 250ml of double distilled water at 50°C, heat and stir until completely dissolved, then add the cefquinol raw material, mix well by ultrasonic wave, low power 400w Ultrasonic for 2 minutes, high power 1000w ultrasonic for 2 minutes, add 0.33g flow aid, mix to form a mixture, then add glutaraldehyde solution, and then use a double-flow spray dryer for spray drying, while requiring magnetic stirring, and spray drying to obtain microspheres.
[0172] Wherein, in the dispersant, the volume ratio of ethanol:isopropanol:dichloro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com