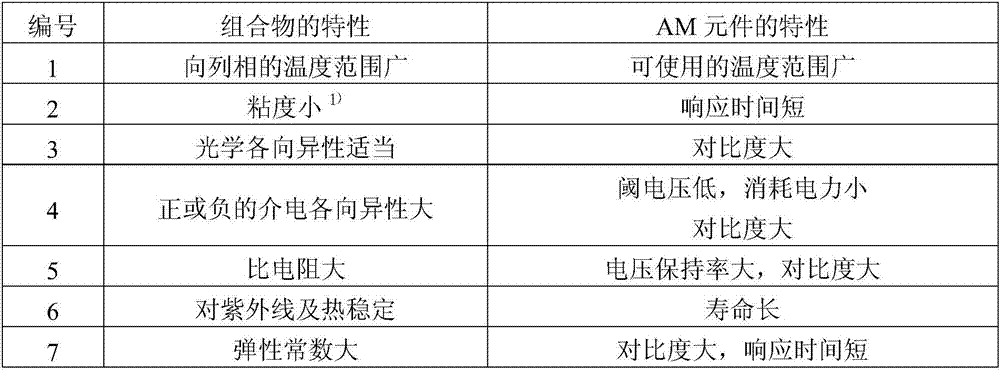
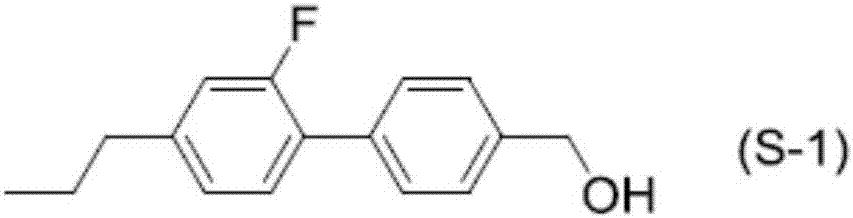
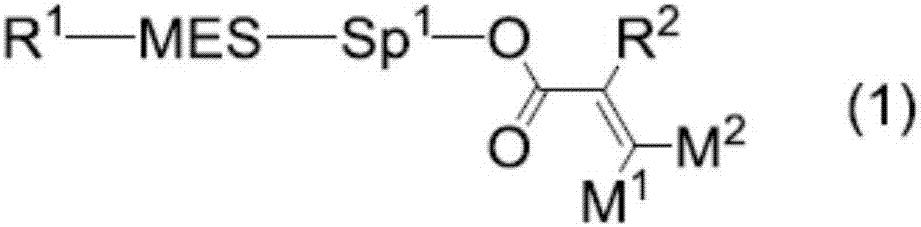
Polymerizable polar compound, liquid crystal composition, and liquid crystal display element
A compound and carbon number technology, used in the field of polymeric polar compounds, can solve the problem of insufficient voltage retention, and achieve the effects of appropriate optical anisotropy, high stability and low threshold voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0390] The present invention will be described in more detail using examples (including synthesis examples and use examples). The present invention is not limited by these Examples. The present invention includes a mixture of the composition of Example 1 and the composition of Example 2. The present invention also includes mixtures prepared by mixing at least two of the compositions of the use examples.
[0391] 1. Examples of compound (1)
[0392] Compound (1) was synthesized according to the procedures shown in the examples. Unless otherwise specified, the reaction was performed under a nitrogen atmosphere. Compound (1) was synthesized according to the procedures shown in Example 1 and the like. The synthesized compounds are identified by nuclear magnetic resonance (Nuclear Magnetic Resonance, NMR) analysis and other methods. The properties of compound (1), liquid crystal compound, composition, and device were measured by the following methods.
[0393]NMR analysis: ...
Synthetic example 1
[0440] Synthesis of compound (1-4-2)
[0441] [chem 93]
[0442]
[0443] Step 1
[0444] Compound (T-1) (25.0 g), acrylic acid (7.14 g), DMAP (1.21 g) and dichloromethane (300 ml) were put into a reactor, and cooled to 0°C. A solution of DCC (24.5 g) in dichloromethane (125 ml) was slowly added dropwise thereto, returned to room temperature and stirred for 12 hours. After removing the insoluble matter, the reaction mixture was poured into water, and the aqueous layer was extracted with dichloromethane. The organic layer formed at the same time was washed with water, and dried over anhydrous magnesium sulfate. The solution was concentrated under reduced pressure, and the residue was purified by silica gel chromatography (volume ratio, heptane:toluene=2:1). Furthermore, it purified by recrystallization from solmix (registered trademark) A-11, and compound (T-2) (11.6g; 38%) was obtained.
[0445] Step 2
[0446] Put paraformaldehyde (2.75g), DABCO (4.62g) and water (40...
Synthetic example 2
[0452] Synthesis of compound (1-4-22)
[0453] [chem 94]
[0454]
[0455] Step 1
[0456] Compound (T-4) (42.5 g; 65%) was obtained by the same method as in the second step of Synthesis Example 1, using compound (T-3) (50.0 g) as a raw material.
[0457] Step 2
[0458]Compound (T-4) (42.5g), imidazole (24.5g) and dichloromethane (740ml) were put into the reactor and cooled to 0°C. A dichloromethane (110 ml) solution of t-butyldimethylsilyl chloride (54.1 g) was slowly added dropwise thereto, returned to room temperature, and stirred for 12 hours. The reaction mixture was poured into water, and the aqueous layer was extracted with dichloromethane. The organic layer formed at the same time was washed with water, and dried over anhydrous magnesium sulfate. The solution was concentrated under reduced pressure, and the residue was purified by silica gel chromatography (volume ratio, heptane:ethyl acetate=10:1) to obtain compound (T-5) (79.8 g; 100%).
[0459] Step 3
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com