Yeast recombinant collagen
A technology for recombinant collagen and collagen, which is applied in the production field of yeast recombinant collagen, can solve the problem of low collagen expression, and achieve the effect of simple operation and product safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
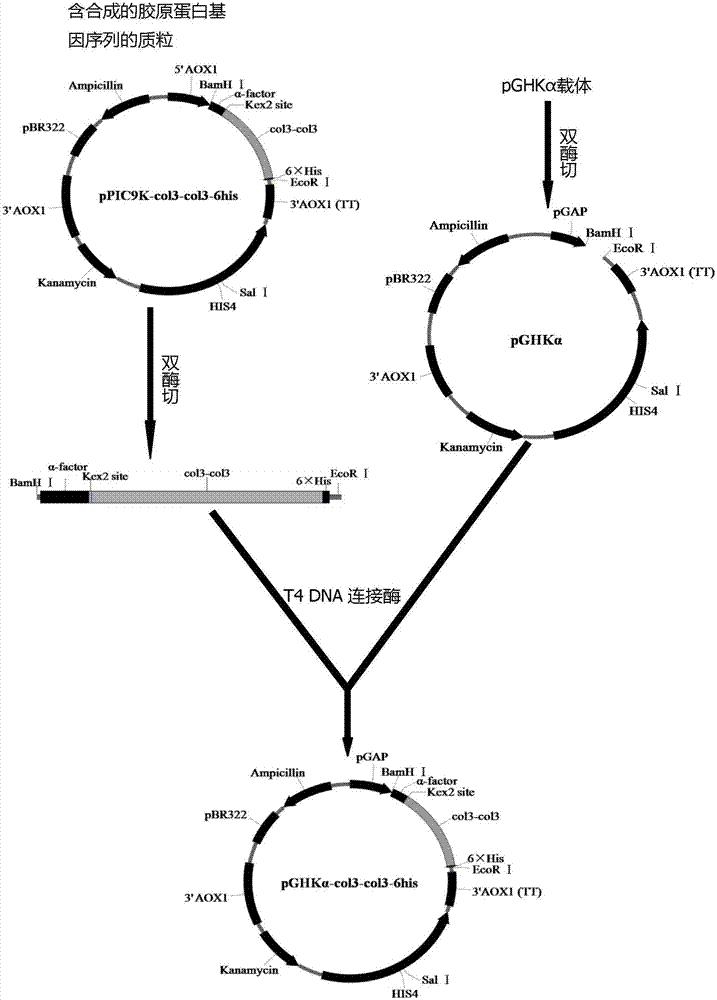
[0072] Example 1 The acquisition of recombinant constitutive expression plasmids (such as figure 1 shown)
[0073] (1) Optimize gene synthesis
[0074] According to the amino acid sequence of collagen obtained by the method disclosed in Chinese patent 201310033299.6 and the gene sequence of type III collagen peptide registered in Genbank, referring to the preferred codons of Pichia pastoris, the DNASTAR software was used without changing the original amino acid sequence. Optimize the gene sequence, and then obtain the optimized gene sequence through whole gene synthesis, and introduce the coding sequence of Kex2 restriction site at the front end, and add TAA site and EcolRI restriction site at the 3′ end of the optimized gene. Synthesized by the method to obtain the plasmid pPIC9k-col3-col3-6his containing the target gene sequence.
[0075] (2) Construction of recombinant expression plasmids
[0076]In the related experiments of the present invention, the plasmid pPIC9k-col...
Embodiment 2
[0077] Example 2 The acquisition of recombinant Pichia pastoris engineering bacteria
[0078] (1) Linearization of recombinant constitutive expression plasmid pGHKα-col3-col3-6his
[0079] Extract the recombinant plasmid pGHKα-col3-col3-6his, digest it with restriction endonuclease SalI at 37°C, and then use 0.7% agarose gel electrophoresis to detect whether it is completely cut. After it is completely cut, use gel to recover The kit processes the enzyme cutting solution, recovers the linearized plasmid, and desalts it.
[0080] (2) Preparation of Pichia pastoris SMD1168 competent cells
[0081] ①Pick a single colony of yeast SMD1168, inoculate it into a Erlenmeyer flask containing 5mL of YPD liquid medium, and cultivate overnight at 30°C and 220rpm with shaking;
[0082] ② Take 50 μL of the overnight culture and inoculate it into a 500 mL Erlenmeyer flask containing 50 mL of fresh YPD liquid medium, culture at 30°C and 220 rpm with shaking overnight, until the OD 600 The v...
Embodiment 3
[0104] Example 3 Yeast recombinant collagen production
[0105] ⑴Use YPD to cultivate recombinant Pichia pastoris engineering bacteria based on overnight cultivation at 30°C and 220rpm;
[0106] (2) Take an appropriate amount of overnight culture and insert it into BMDY medium, so that the OD 600 =1.0-2.0, cultured with shaking at 28°C and 220 rpm for 4 days.
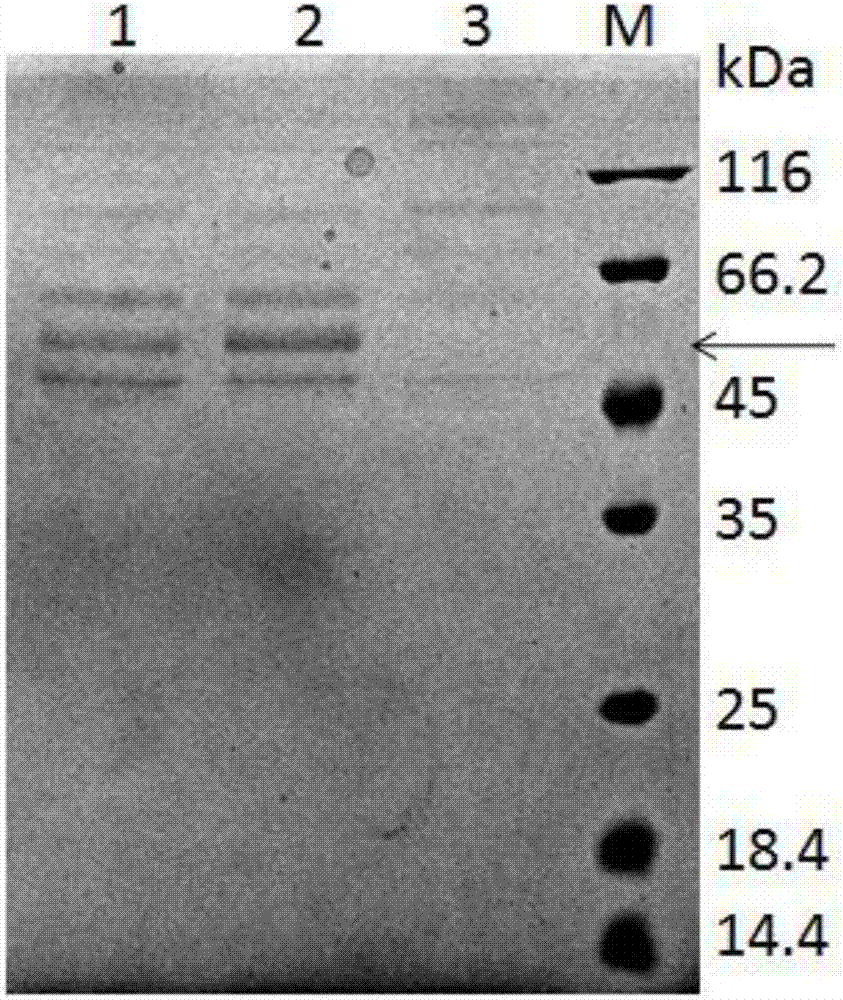
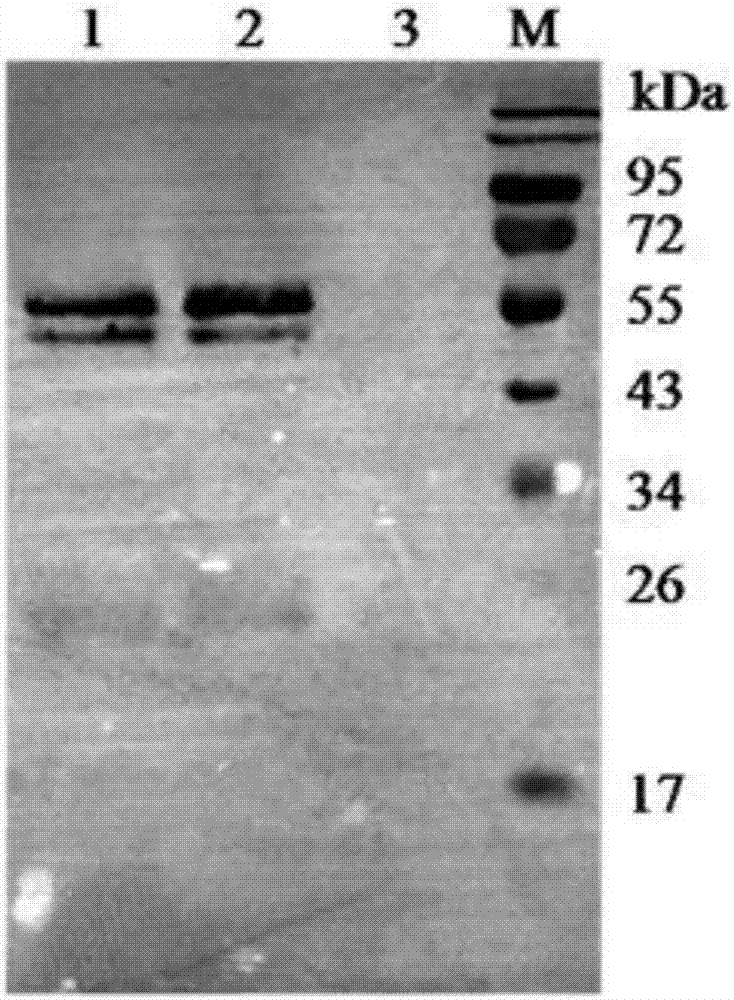
[0107] (3) After the fermentation culture, centrifuge at 5000×g for 20 min at 4°C, and collect the supernatant. Take a small amount of supernatant for SDS-PAGE analysis and detection, the results are as follows figure 2 As shown, according to the His tag, Western Blot analysis was performed to confirm that the main protein band was the target protein, and the results were as follows image 3 shown;
[0108] (4) Add 5 to 7 times the volume of pure water to the supernatant, and then concentrate it to 20% of the initial volume by ultrafiltration;
[0109] (5) Add 100 μL 1×Ni-NTA binding buffer to 1 mL of concentrated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com