Chimeric antigen receptor for identifying carcino-embryonic antigens and application of chimeric antigen receptor
A technology of chimeric antigen receptor and carcinoembryonic antigen, which is applied in the field of genetic engineering, can solve the influence of no report showing the stability of chimeric antigen receptor cells and the efficacy of CEA solid tumors, and the stability of antigen chimeric receptors is not very good. It can improve the ability to proliferate and kill tumors, improve the ability to remove tumor cells, and achieve high efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
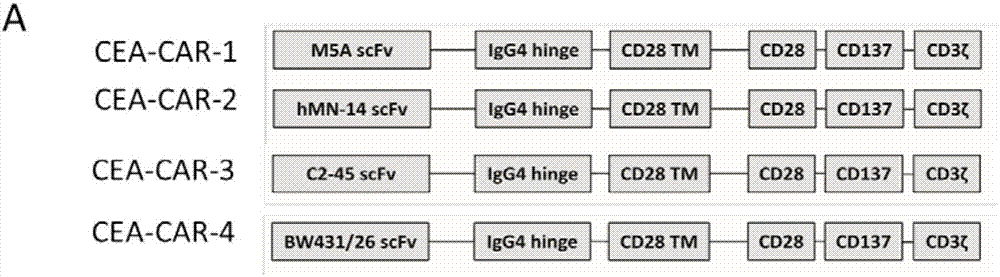
[0066] Example 1 Construction of chimeric antigen receptor virus containing scFV of M5A, hMN-14, C2-45 and BW431 / 26
[0067] In order to prove that the anti-CEA CAR we screened has more advantages than the currently used anti-CEA CAR and other anti-CEA scFV CARs, it is necessary to simultaneously construct scFV-CARs of different anti-CEA humanized monoclonal antibodies named CEA-CAR- 1. CEA-CAR-2, CEA-CAR-3 and CEA-CAR-4, as follows:
[0068] 1 Synthesis of gene sequences of chimeric antigen receptors targeting carcinoembryonic antigen containing different scFVs
[0069] Synthesize different single chain antibodies ScFv containing leader peptide (also known as signal peptide) (LP for short), anti-human CEA antigen, IgG4 hinge region (IgG4 for short), CD28 transmembrane region or CD8 transmembrane region (TM for short) , whose structure is as figure 1 shown.
[0070] 2 Construction of lentiviral vector expressing chimeric antigen receptor
[0071] The following primers were...
Embodiment 2
[0093] Example 2 scFV and target antigen (CEA) affinity determination
[0094] The aforementioned 4 scFVs labeled with His tag were cloned into plasmid vector and transfected into HEK293 cells. The transfected cells were cultured for 6 days and all supernatants were collected. The supernatants were filtered through a 0.22um filter and purified by nickel column affinity chromatography. The scFV-His in the serum was purified, and the purity of scFV-His reached 95%. The concentration of scFV-His was determined by Bradford; the affinity of different scFVs to CEA was determined by ForteBio The RED96 interaction analyzer (Pall, CA, U.S.) was used for inspection, and the experimental steps were carried out according to the instructions. The anti-human IgG-Fc (AHC) biosensor was purchased from Pall Forté Bio, 1512121 according to the instructions; the concentration of recombinant CEA-Fc was 15ug / ml, and the product number was 11077-H03H-50 from Sino Biological Inc. Incubated in the ...
Embodiment 3
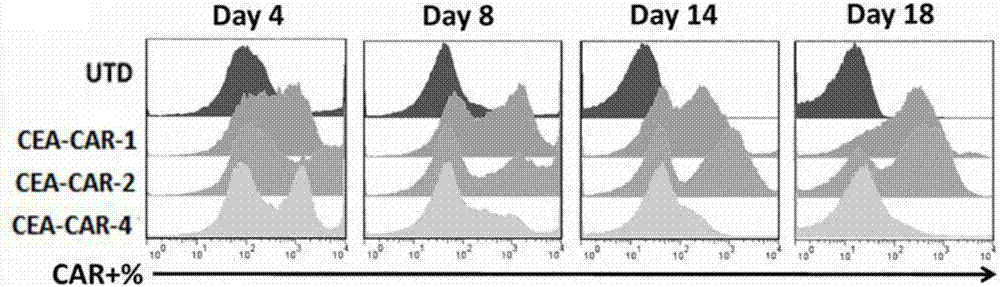
[0095] Example 3 Detection of Chimeric Antigen Receptor Maintenance in T Cells
[0096] 1 Isolation of human peripheral blood mononuclear cells
[0097] Collect about 60ml of peripheral blood with blood collection tubes with anticoagulant, divide into 30ml of 50ml centrifuge tubes, add 7.5ml of hydroxyethyl starch to dilute; settling naturally at room temperature (18-25℃) for about 30min, collect upper plasma, The collected upper plasma was centrifuged at 1400 rpm / min for 15 min; then the precipitate was resuspended with normal saline, added to the lymphocyte separation medium at a volume ratio of 1:1, gradient centrifuged at 400 g / min, and the deceleration rate of the centrifuge was set to 1 After centrifugation, the centrifuge tube is divided into: first layer: plasma layer; second layer: annular milky white lymphocyte layer; third layer: transparent separation liquid layer; fourth layer: red blood cell layer; Take the second white lymphocyte layer and wash it twice with no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com