Polypeptides
A technology for combining fragments and antigens, applied in the field of polypeptides, which can solve the problems of inability to mediate Fc effector functions, poor stability of single-chain fragments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[1765] Example 1 – Generation and Manufacturability Evaluation of 20 Variants in an IgG-Fab Architecture
[1766] Materials and methods
[1767] design
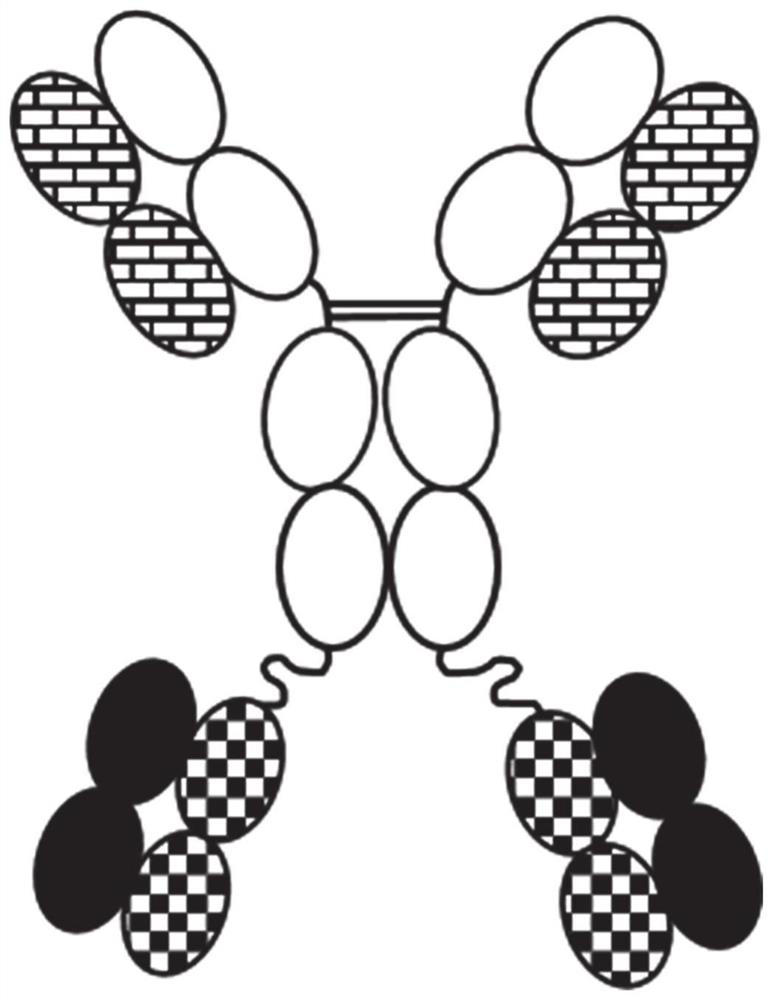
[1768] A novel bispecific construct consisting of immunoglobulin (IgG) coupled to two Fab fragments via the C-terminal end of IgG was engineered into 20 different variants and the polypeptide linker between the N-terminal end of the Fab fragment light chain ( figure 1 ). Variants were generated using different combinations of mutations in the interface between VH-CH1 and VL-CK. The mutations used are well known in the public domain.
[1769] manufacturability
[1770] Bispecific antibodies were expressed in varying volumes of 600 μL–2 L using transient HEK293 and Expi293 HEK (Life Technologies) cultures according to the manufacturer's instructions. Using NGC system (BioRad), Bispecific proteins were purified from supernatants using the Avant system (GE Healthcare) or Predictor MabSelectSure 50 [mu]l 96-well plates (GE...
example 2
[1791] Example 2 - Stability and Solubility
[1792] Materials and methods
[1793] thermal stability
[1794] Melting temperatures were measured with a UNCle system (UNchained labs). Intrinsic fluorescence was measured during a linear ramp from 20°C to 95°C at a rate of 0.4°C / min. Data analysis was performed using UNcle Analysis software version 2.0 with default settings.
[1795] stability
[1796] Incubate samples in non-optimized buffer (PBS) at low (<1 mg / mL) or high (10 mg / mL) protein concentration at 2-8°C, room temperature, and 40°C for 1, 2, and 4 weeks or 1 1 week and 2 weeks, or 3 cycles of freeze-thaw. Protein degradation was measured by SE-HPLC, SDS-PAGE, A280, double ELISA and by visual inspection.
[1797] Shear Stress Stability
[1798] Duplicate samples were subjected to shear stress on a 96-well plate shaker MixMate (Eppendorf) with vigorous stirring at 2000 rpm for at least 30 minutes. Protein precipitates were removed by centrifugation at 3000 g for 1...
example 3
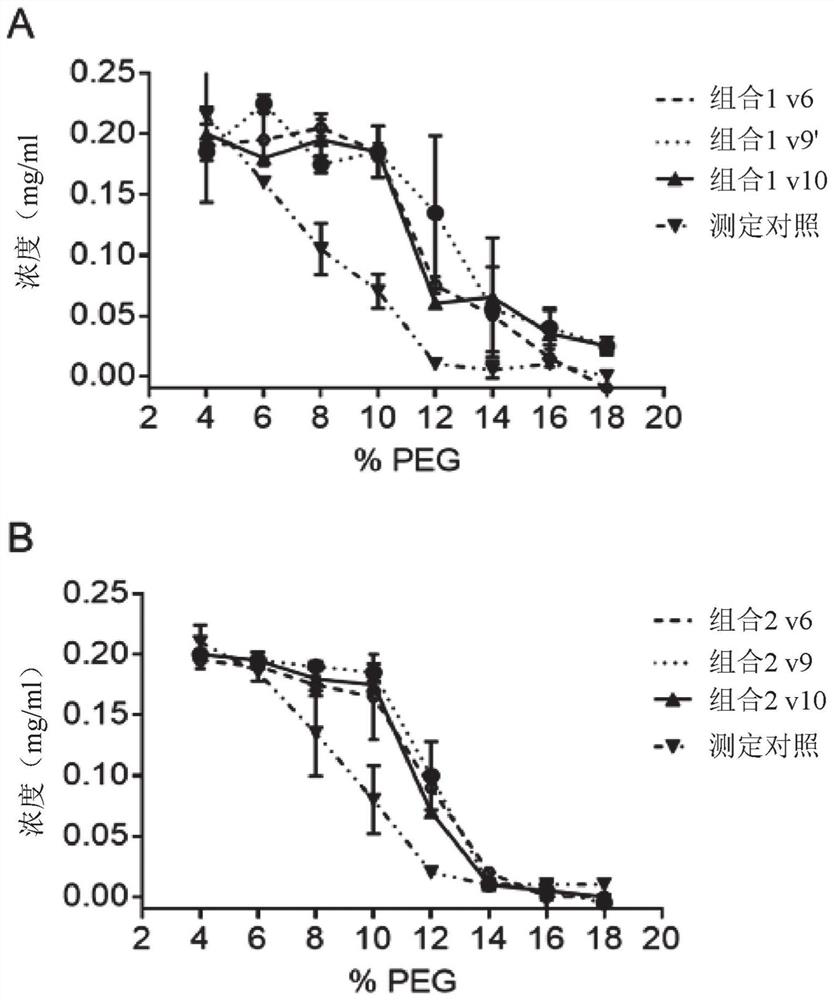
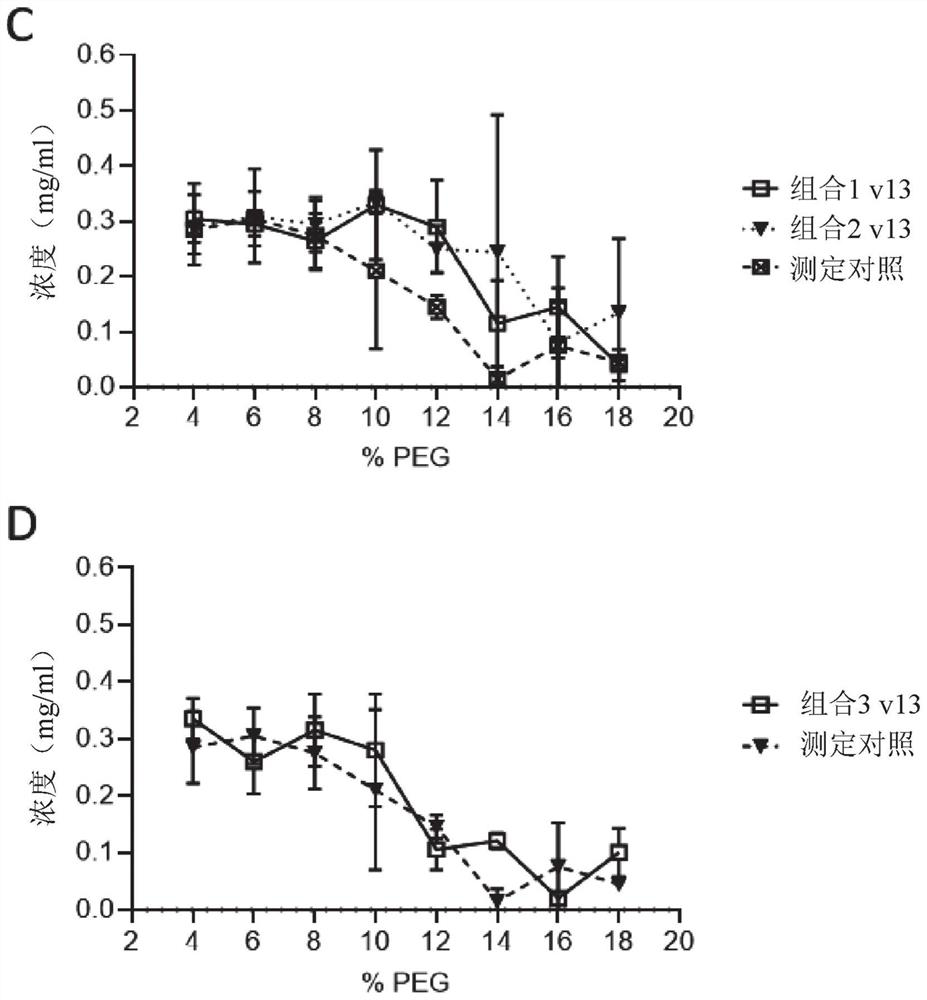
[1817] Example 3 – Combination Study
[1818] Materials and methods
[1819] double ELISA
[1820] Plates were coated with 0.5 μg / mL antigen (Ag1 or Ag2) in PBS overnight at 4°C. After washing in PBS / 0.05% Tween20 (PBST), plates were blocked with PBS / 2% BSA for at least 30 minutes at room temperature and then washed again. Serially diluted samples in PBS / 0.5% BSA were then added and allowed to bind for at least 1 hour at room temperature. After washing, plates were incubated with 0.5 μg / mL biotinylated Ag1 or Ag2 (one of the antigens not used for coating) for at least 1 hour at room temperature. Double-complexed bsAbs with Ag1 and Ag2 were detected with HRP-labeled streptavidin. SuperSignal Pico Luminescent was used as the substrate, and the luminescent signal was measured using Fluostar Optima.
[1821] Octet
[1822] Kinetic measurements were performed using the Octet RED96 platform (ForteBIo). Biotinylated or Fc-labeled antigens were coupled to streptavidin or amine-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com