A method for the synthesis of phenol by highly selective oxidation of benzene
A technology of high selectivity and synthesis method, applied in the field of high-selectivity oxidation of benzene to synthesize phenol, can solve the problems of low phenol yield and the like, and achieve the effects of high selectivity, low equipment corrosion and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The present invention prepares the photocatalyst by changing the molar ratio of cadmium tungstate and bismuth tungstate in the cadmium tungstate-bismuth tungstate composite photocatalyst:
[0025] Preparation of cadmium tungstate nanorods: Dissolve 1.234g of cadmium nitrate and 0.240g of ethylenediamine solution in 20mL of deionized water, stir vigorously for 10min and mix to form solution A; add 20mL of aqueous solution B containing 1.316g of sodium tungstate drop by drop Add to solution A, stir and mix well, adjust the pH to 7 with nitric acid, and continue stirring for 20 minutes to obtain solution C. The resulting solution C was then crystallized at 160°C for 20 hours. Cool and centrifuge, wash with deionized water three times, and dry at 80°C for 4 hours to obtain cadmium tungstate;
[0026] Preparation of composite materials: Dissolve 0.015g sodium tungstate and 0.040g bismuth nitrate in 30mL ethylene glycol solution respectively, stir well and mix to form soluti...
Embodiment 2~5
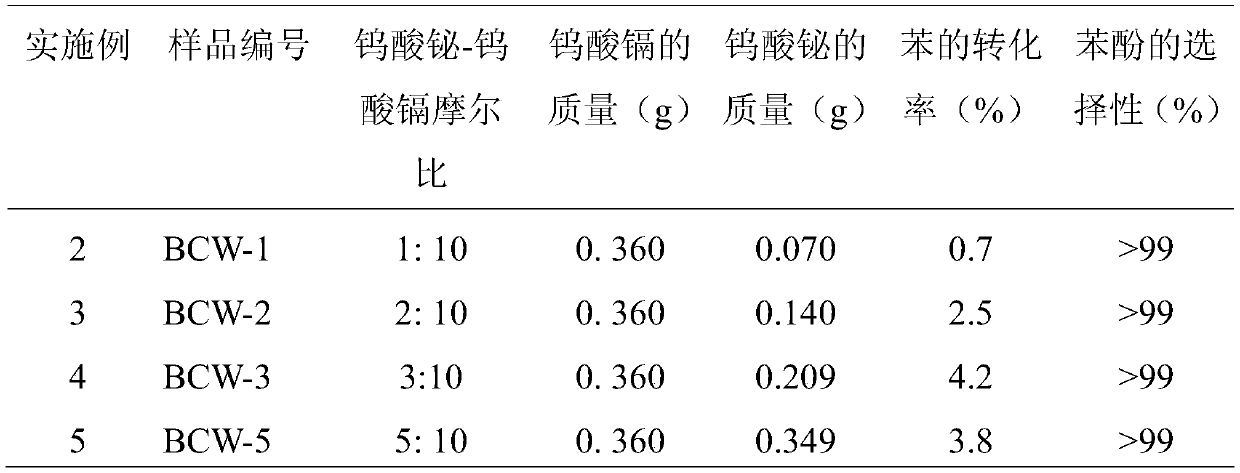
[0030] For cadmium tungstate and bismuth tungstate composite photocatalysts with different molar ratios, the operation steps are similar to those in Example 1, only the amount of sodium tungstate and bismuth nitrate in the composite material is changed, and the rest of the conditions are unchanged, and the sample number BCW-1, BCW-2, BCW-3, BCW-4, BCW-5. See Table 1 for the composite catalyst conditions and reaction results prepared in Examples 2-5.
[0031] Table 1. Reaction results of bismuth tungstate-cadmium tungstate composite photocatalysts with different molar ratios
[0032]
[0033] As can be seen from Table 1, different benzene conversion rates are obtained under different molar ratios of cadmium tungstate and bismuth tungstate, wherein when the ratio of bismuth tungstate-cadmium tungstate is 4:10, the conversion rate of catalyzed benzene is 5.8%, and that of phenol The selectivity is greater than 99%, and the photocatalytic effect is very good.
Embodiment 6~10
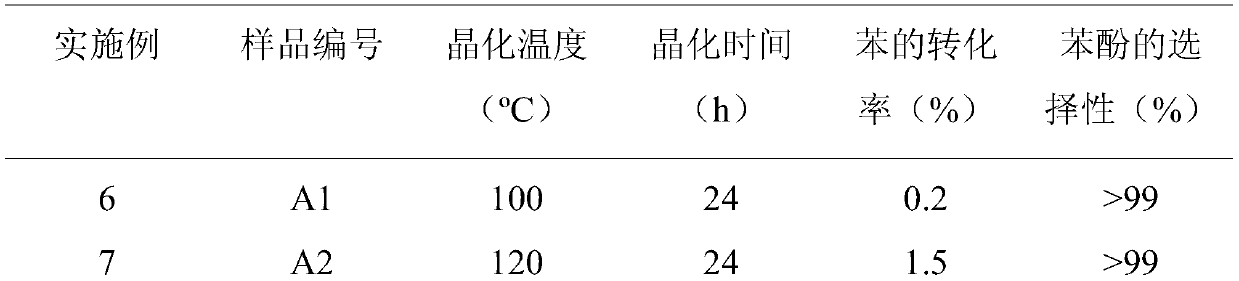
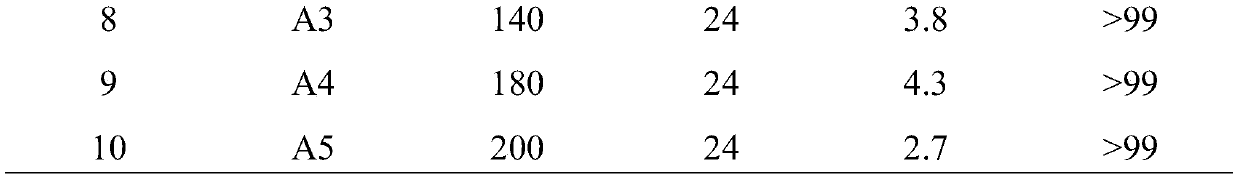
[0035] According to the steps of Example 1 with the best effect, the remaining conditions remain unchanged (the molar ratio of bismuth tungstate and cadmium tungstate is 4:10), only the crystallization temperature during the preparation of the composite photocatalyst is changed to 100°C respectively , 120°C, 140°C, 180°C, 200°C, and the sample numbers are A1, A2, A3, A4, A5. See Table 2 for the composite catalyst conditions and reaction results prepared in Examples 6-10.
[0036] Table 2. Reaction results of bismuth tungstate-cadmium tungstate composite photocatalysts crystallized at different temperatures
[0037]
[0038]
[0039] It can be seen from Table 2 that the conversion rate of benzene obtained at different crystallization temperatures is compared with that of Example 1. The conversion rate of benzene is the highest when the crystallization temperature is 160° C., which has the best photocatalytic effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com